Common menu bar links
Breadcrumb Trail
ARCHIVED - Assisted Human Reproduction Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Minister's Message

Helping Canadians to maintain and improve their health remains a top priority of the Government of Canada. As this 2010-11 Report on Plans and Priorities demonstrates, Assisted Human Reproduction Canada (AHRC) is actively advancing this agenda by protecting and promoting the health, safety and dignity of Canadians who use, or are born of, assisted human reproduction (AHR) technologies.
As the federal regulatory agency responsible for overseeing assisted reproduction and related research, AHRC is charged with building strong networks with key stakeholders working in this area. It works with professional organizations, governments, academics and non-governmental organizations across Canada and around the world. Working together, they establish needs and priorities and, in some cases, identify common concerns and solutions in this ever-evolving field.
I am proud of AHRC's progress in this regard. While regulations to the Assisted Human Reproduction Act continue to be developed by Health Canada, the Agency is expanding our knowledge of assisted reproduction. Equally valuable, it is ensuring relevant information is made widely available to Canadians through its public outreach and education efforts. It also continues to put in place the systems and processes necessary to safeguard the health and safety of Canadians, and is constantly monitoring the latest trends in this area in order to provide advice to the Government of Canada on these often challenging matters.
The forward-looking activities outlined in this report reinforce AHRC's commitment to ongoing progress. In the coming year, it will take action on a number of fronts to continue to meet the needs and expectations of Canadians who look to assisted human reproduction technologies to build their families.
The Honourable Leona Aglukkaq
Minister of Health
President's Message

With each passing year more and more Canadians are turning to the Agency as a trusted centre of expertise on assisted reproduction. This is due in large part, to the dedication and excellence of the team here at Assisted Human Reproduction Canada, and the generosity and collaboration of our Science Advisory Panel and other professionals who generously volunteer their time.
This year, our focus will be given to establishing the infrastructure required to implement the Assisted Human Reproduction Act regulations as they come into force, including collaborative agreements with key stakeholders and government partners as well as systems development and data collection.
Given the rights and dignity involved of those Canadians who use AHR to build their families, the Agency remains diligent and responds to possible violations of the Act brought to our attention. AHRC can, and will, take appropriate compliance action when necessary.
We continue to support important research in priority areas for discovery - from the epidemiology of infertility to psycho-social research. I am proud of the Agency's leadership in examining global challenges such as health and safety concerns surrounding multiple births and cross-border AHR treatment and care. Much of this excellent work is carried out collaboratively with our domestic and international partners. For example, in partnership with key stakeholders, AHRC hosted a Multiple Births Roundtable to bring health professionals together to discuss shared concerns. As a result of the roundtable, a national framework for the prevention of multiple births due to fertility treatments was developed. Professional organisations are exploring ways to implement priority activities and strategies identified at the workshop.
We will carry on delivering the Agency's mandate to protect and promote the health, safety, dignity and rights of Canadians in relation to assisted human reproduction. AHRC will continue to work with Statistics Canada and the Canadian Institutes of Health Research to develop the evidence-base required to support policy and regulatory decision-making. A further objective for the coming year is the development of a strategy to inform Canadians about the risk factors for infertility.
Enhanced information offerings on the broad range of AHR issues of interest to all Canadians remains a key priority for the Agency. In addition to preparing relevant information on numerous topics, AHRC is continually improving its web presence to keep Canadians better informed about our activities and AHR generally.
As momentum builds and measurable progress is made, I remain confident that a positive course has been set for our Agency's ongoing success in the future.
Dr. Elinor Wilson, President
Assisted Human Reproduction Canada
Health Portfolio Overview
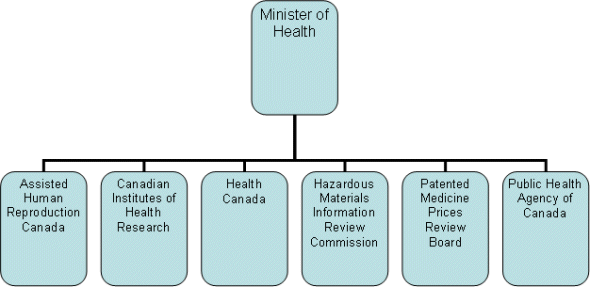
The Minister of Health is responsible for maintaining and improving the health of Canadians. These efforts are supported by the Health Portfolio, which includes Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board and Assisted Human Reproduction Canada. Each member of the Portfolio prepares its own Report on Plans and Priorities.
Section I - Agency Overview
1.1 Summary Information
Raison d'être
The Assisted Human Reproduction Agency of Canada (AHRC) was established under the authority of the Assisted Human Reproduction Act (AHR Act). The Act seeks to protect and promote the health, safety, dignity and rights of those who use assisted human reproduction (AHR) technologies; prohibits unacceptable activities such as human cloning, sex-selection or commercialization of human reproductive capabilities, and places controls over AHR-related research. The Agency is responsible for issuing and reviewing licences, developing and managing a health reporting information registry, establishing a health surveillance system, and carrying out inspections and compliance and enforcement activities related to activities controlled under the Act. The Agency is also a centre of expertise and a focal point of AHR information for policy makers, health professionals and all Canadians.
Responsibilities
Assisted Human Reproduction Canada (AHRC) is the federal regulatory agency responsible for protecting and promoting the health, safety, dignity and rights of Canadians who use or are born of assisted human reproduction technologies.
AHRC is also responsible for fostering an environment in which ethical principles are applied in all matters relating to assisted human reproduction, while allowing scientific advances that benefit Canadians.
AHRC's mandate and responsibilities are set out in the Assisted Human Reproduction Act. The Agency's key responsibilities include:
- implementing and administering the licencing framework for controlled activities, including AHR procedures and related research;
- developing an inspection strategy to ensure compliance with the AHR Act and its regulations;
- developing and maintaining a national Personal Health Information Registry (PHIR) on AHR that can become a key component of a more comprehensive AHR health surveillance strategy;
- becoming a centre of expertise on AHR by collecting and disseminating public information;
- communicating with and engaging stakeholders on AHR issues; and
- advising the Minister of Health on AHR issues.
Strategic Outcome
In order to effectively pursue its mandate, the Agency aims to achieve the following strategic outcome:
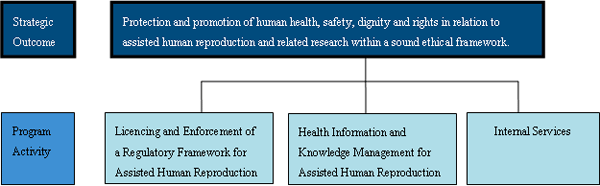
Protection and promotion of human health, safety, dignity and rights in relation to assisted human reproduction and related research within a sound ethical framework.
Program Activity Architecture
The chart bellow illustrates Assisted Human Reproduction Canada's complete framework of program activities, which roll up and contribute to progress toward the Agency's strategic outcome.
PAA Crosswalk
The Strategic Outcome has been revised to reflect the importance of ethical considerations in the Agency's work, and Program Activities have been broadened by removing the word "technologies" from Assisted Human Reproduction within the PA titles. The word "technologies" may be viewed as limiting activities of the Agency to those that are procedural/technical, as opposed to the whole area of Assisted Human Reproduction. Non- technological activities, such as administering medication, or the provision of information related to infertility risk factors (which is specifically mentioned in the Act), are more generally related to AHR.
1.2 Planning Summary
| 2010-11 | 2011-12 | 2012-13 |
|---|---|---|
| 10.5 | 10.5 | 10.5 |
The table above summarizes AHRC's total planned spending for the next three fiscal years. Note that the Agency received no funding under Canada's Economic Action Plan.
| 2010-11 | 2011-12 | 2012-13 |
|---|---|---|
| 44 | 44 | 44 |
The table above summarizes AHRC's total planned human resources for the next three fiscal years.
Summary Table
| Performance Indicators | Targets |
|---|---|
| As the regulations come into force, develop operational guidelines to administer the Assisted Human Reproduction Act and its associated regulations. | In advance of the regulations coming into force:
|
| Program Activity | Forecast Spending ($ millions) |
Planned Spending ($ millions) |
Alignment to Government of Canada Outcomes | ||
|---|---|---|---|---|---|
| 2009-10 | 2010-11 | 2011-12 | 2012-13 | ||
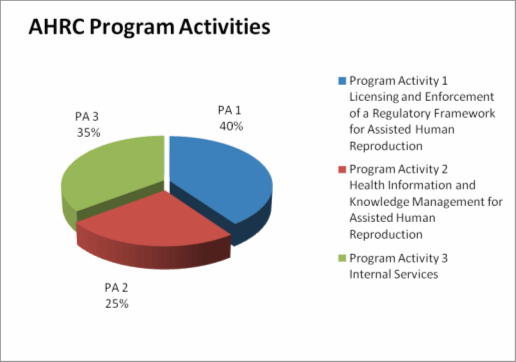
| Licencing and Enforcement of a Regulatory Framework for Assisted Human Reproduction | 4.2 | 4.2 | 4.2 | 4.2 | Healthy Canadians |
| Health Information and Knowledge Management for Assisted Human Reproduction | 2.6 | 2.6 | 2.6 | 2.6 | Healthy Canadians |
| Internal Services | 3.7 | 3.7 | 3.7 | 3.7 | |
| Total | 10.5 | 10.5 | 10.5 | 10.5 | |
Contribution of Priorities to Strategic Outcome
| Operational Priorities | Type | Links to Strategic Outcome | Description |
|---|---|---|---|
| To contribute to the development of AHR regulations by Health Canada | Previously committed to | SO 1 | Health Canada is in the process of developing regulations under the AHR Act that AHRC will administer. The Agency is working to actively contribute to Health Canada's regulatory development process, which will establish the regulatory framework for the delivery of the AHRC mandate. |
| To develop the capacity in AHRC to implement the regulations (once developed) | Previously committed to | SO 1 | The Agency is putting in place the systems and processes required to implement the regulations currently under development by Health Canada, particularly those related to licencing, inspections, and the Personal Health Information Registry. |
| National and International Collaboration | New | SO 1 | Develop strategic relationships among national and international organizations to: facilitate information exchange; and identify and address issues of mutual concern. |
| Management Priorities | Type | Links to Strategic Outcome | Description |
|---|---|---|---|
| Internal Management | Previously committed to | SO 1 | The Agency has developed its planning and reporting instruments, including Board of Directors strategic planning, integrated Agency-level business, and human resources planning and reporting and will continue to implement it as it evolves. Planning and reporting will be aligned with the Program Activity Architecture, with increased emphasis on performance measurement and data collection and a Performance Management and Evaluation Plan (PMEP). |
| Human Resources | Previously committed to | SO 1 | AHRC is establishing its capacity in the specific areas needed to administer the regulations, as well as the processes and systems to support that role. As the Agency evolves, it will need to attract additional qualified resources in specialized areas to augment its core capacities, in view of the scheduled re-location of the temporary headquarters from Ottawa to Vancouver. |
| Financial management | New | SO 1 | The Agency continues to build an Internal Financial Control framework to encompass financial management, risk management, financial delegation and training as well as contracting. |
Risk Analysis
AHRC has an interim corporate risk management framework in place until the full regulatory framework is implemented. As part of that framework, the Agency continues to review, monitor and manage its risks annually and updates plans to manage those risks, and to integrate a risk monitoring and reporting process into its planning cycle.
Assisted human reproduction continues to change at a rapid pace. This change carries health, ethical and social implications for Canadians. The Agency's Science Advisory Panel, a committee of the Board of Directors, provides AHRC with advice from recognised experts in the field of Assisted Human Reproduction, and keeps the Agency abreast of new developments and related implications. The Agency continues to expand its collaborations and partnerships with key stakeholders in the scientific community, and with federal health portfolio partners to identify issues and gaps and to lay the groundwork for an effective regulatory system.
While the regulations are being developed by Health Canada, AHRC is working to develop the infrastructure (i.e., policies, procedures and systems) to effectively and efficiently manage its operations, including the specialized systems that will be required to implement the regulatory process once the regulations come into force. The important systems required to administer the regulations and Personal Health Information Registry are being developed in a manner consistent with the phased approach of the development of the regulations by Health Canada. Efforts are being undertaken to ensure the appropriate analysis and safeguarding of the personal health information the Agency will eventually need to collect. The Agency will continue to consult the Office of The Privacy Commissioner.
AHRC recognizes the importance of our various stakeholder groups to successful regulation of AHR. The Agency's stakeholder outreach strategy continues to build on its successful efforts to identify and ensure engagement of all appropriate stakeholders.
Expenditure Profile
For the 2010-11 fiscal years, Assisted Human Reproduction Canada plans to spend $10.5 million to meet the expected results of its program activities and contribute to its strategic outcome. The figure below illustrates Assisted Human Reproduction Canada's spending trend from 2006-07 to 2012-13.
AHRC Spending Trend ($millions)
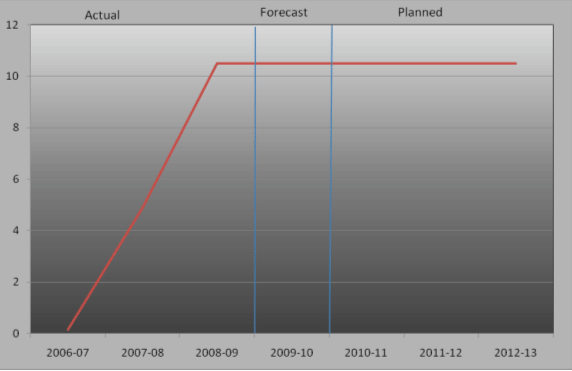
For the 2006-07 to 2009-10 periods, the total spending includes all Parliamentary appropriations (Main Estimates, Supplementary Estimates and Treasury Board Vote 15) as well as carry- forward adjustments. For the 2010-11 to 2012-13 periods, the total spending corresponds to the planned spending. Supplementary funding and carry forward adjustments are not reflected.
From 2007-08 to 2009-10, AHRC's spending has increased since the establishment of the Agency, additional staff were hired and the infrastructure necessary to fulfill its mandate has been put in place. Until the regulations are complete, the Agency in 2010-11 will focus its activities on those related to the preparation of systems required to support the regulations. The Agency will continue to monitor and enforce compliance with the AHR legislative and regulatory framework and build its capacity to protect and promote the health and safety of donors, patients and offspring born of assisted human reproduction technologies. Assisted Human Reproduction Canada will continue to fullfil its outreach mandate through public and professional educational activities. The focus will shift to operations in delivering its regulatory mandate once the regulations are in place.
The figure below displays the allocation of AHRC's funding by program activity for 2010-11.
2010-11 Allocation of Funding by Program Activity
Voted and Statutory Items
This table illustrates the way in which Parliament approved AHRC resources, and shows the changes in resources derived from supplementary estimates and other authorities, as well as how funds were spent.
| Vote # or Statutory Item (S) | Truncated Vote or Statutory Wording | 2009-10 Main Estimates ($ millions) |
2010-11 Main Estimates ($ millions) |
|---|---|---|---|
| 15 | Operating expenditures | 9.9 | 9.9 |
| (S) | Contributions to employee benefit plans | 0.6 | 0.6 |
| Total | 10.5 | 10.5 | |
