Common menu bar links
Breadcrumb Trail
ARCHIVED - Assisted Human Reproduction Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Minister's Message

I am pleased to present the 2009–10 Report on Plans and Priorities for Assisted Human Reproduction Canada (AHRC), which highlights the Agency’s progress in ensuring that the gift of life made possible through the use of reproductive technologies happens in a safe, healthy and dignified way.
AHRC was created to protect and promote the rights and interests of Canadians who use or are born of assisted human reproduction technologies, and to foster the application of ethical principles in the use and development of those technologies. As the federal regulatory agency charged with overseeing assisted human reproduction (AHR) and related research, AHRC is also a centre of expertise and a focal point of AHR information for policy makers, health professionals and Canadians.
AHRC has accomplished a great deal in its short history. The Agency is actively establishing the systems and processes required to safeguard the health and safety of Canadians who use reproductive technologies to build their families. AHRC continues to build networks with key stakeholders involved in assisted human reproduction, both across Canada and around the world. AHRC is consulting and collaborating with a broad range of stakeholders to increase the inclusion of, and to give voice to, all Canadians with an interest in this issue.
AHRC’s work is critical to the thousands of Canadians across the country who need help in having the children they want.
I am pleased to present the 2009–10 Report on Plans and Priorities for AHRC, which outlines the Agency’s plans to carry on this valuable work by taking action on multiple fronts to fulfill its mandate to ensure the health, safety, dignity and rights of Canadians building their families through AHR.
The Honourable Leona Aglukkaq
Minister of Health
President's Message

I am proud of the remarkable progress our dedicated team has made in the brief time since Assisted Human Reproduction Canada (AHRC) formally began its operations in February 2007. These successes are due in large part to the hard work of our strong core team that laid the foundation of our organization, and to the energy and enthusiasm of talented new recruits we have added to our roster over the past year. Credit is also due to our stakeholders, who have shared their insights and ideas as we construct the systems and processes needed to implement the regulations once they come into force.
From the outset, we have recognized the necessity of working with the communities we serve to understand their needs and concerns, and to help the Agency establish the regulatory regime in an open, transparent, sensitive and respectful manner. We have also consistently appreciated the importance and benefits of collaboration in advancing common goals.
Our engagement with stakeholders has underscored the crucial importance of the Agency’s role: to protect the health, safety, dignity and rights of individuals who use or are born of reproductive technologies.
Assisted human reproduction (AHR) has profound implications not only for the families directly affected by AHR, but for all of society. Recognizing this, over the coming years we will continue to reach out to our stakeholders and to all Canadians, educating them about the requirements of the Assisted Human Reproduction Act and our regulatory responsibilities flowing from it. AHRC will provide information to the public through a variety of information vehicles, particularly the Web site.
We will seek our stakeholders’ feedback in implementing the regulations and identifying areas of mutual concern where collaboration can lead to advancement and resolution. The Agency will continue to respond to concerns brought to its attention about possible violations of the Act, taking appropriate compliance action. AHRC will also collaborate with the medical community and other stakeholders to develop strategies to address concerns about AHR and multiple births, as well as, maintaining and strengthening our ties to the international AHR community.
I am confident that we will continue to consolidate our achievements and strengthen our capacity as we build AHRC over the next three years.
Dr. Elinor Wilson, President
Assisted Human Reproduction Canada
Health Portfolio Overview
The Minister of Health is responsible for maintaining and improving the health of Canadians. These efforts are supported by the Health Portfolio, which includes Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board and Assisted Human Reproduction Canada. Each member of the Portfolio prepares its own Report on Plans and Priorities.
Section I - Agency Overview
1.1 Summary Information
Raison d’être
The Assisted Human Reproduction Agency of Canada (AHRC) was established under the authority of the Assisted Human Reproduction Act (AHR Act). The Act seeks to protect and promote the health, safety, dignity and rights of those who use assisted human reproduction (AHR) technologies; prohibits unacceptable activities such as human cloning, sex-selection or commercialization of human reproductive capabilities, and places controls over AHR-related research. The Agency is responsible for issuing and reviewing licences, developing and managing a health reporting information registry, establishing a health surveillance system, and carrying out inspections and compliance and enforcement activities related to activities controlled under the Act. The Agency is also a centre of expertise and a focal point of AHR information for policy makers, health professionals and all Canadians.
Responsibilities
Assisted Human Reproduction Canada (AHRC) is the federal regulatory agency responsible for protecting and promoting the health, safety, dignity and rights of Canadians who use or are born of assisted human reproduction technologies.
AHRC is also responsible for fostering an environment in which ethical principles are applied in all matters relating to assisted human reproduction, while allowing scientific advances that benefit Canadians.
AHRC’s mandate and responsibilities are set out in the AHR Act. The Agency’s key responsibilities include:
- implementing and administering the licencing framework for controlled activities, including AHR procedures and related research;
- developing an inspection strategy to ensure compliance with the AHR Act and its regulations;
- developing and maintaining a national Personal Health Information Registry (PHIR) on AHR that can become a key component of a more comprehensive AHR health surveillance strategy;
- becoming a centre of expertise on AHR by collecting and disseminating public information;
- communicating with and engaging stakeholders on AHR issues; and
- advising the Minister of Health on AHR issues.
Strategic Outcome
In order to effectively pursue its mandate, the Agency aims to achieve the following strategic outcome:
Program Activity Architecture
The chart bellow illustrates Assisted Human Reproduction Canada’s complete framework of program activities, which roll up and contribute to progress toward the Agency’s strategic outcome.

1.2 Planning Summary
Financial Resources
| Financial Resources | 2009-10 | 2010-11 | 2011-12 |
|---|---|---|---|
| ($ millions) | 10.5 | 10.5 | 10.5 |
The table above summarizes AHRC’s total planned spending for the next three fiscal years.
Human Resources
| Human Resources | 2009-10 | 2010-11 | 2011-12 |
|---|---|---|---|
| Full-Time Equivalents (FTEs) | 44 | 44 | 44 |
The table above summarizes AHRC’s total planned human resources for the next three fiscal years.
Summary Table
| Performance Indicators | Targets |
|---|---|
| As the regulations come into force, develop operational guidelines to administer the Assisted Human Reproduction Act and its associated regulations. |
In advance of the regulations coming into force:
|
| Program Activity | Expected Results | Planned Spending ($ millions) |
Alignment to Government of Canada Outcomes | ||
|---|---|---|---|---|---|
| 2009-10 | 2010-11 | 2011-12 | |||
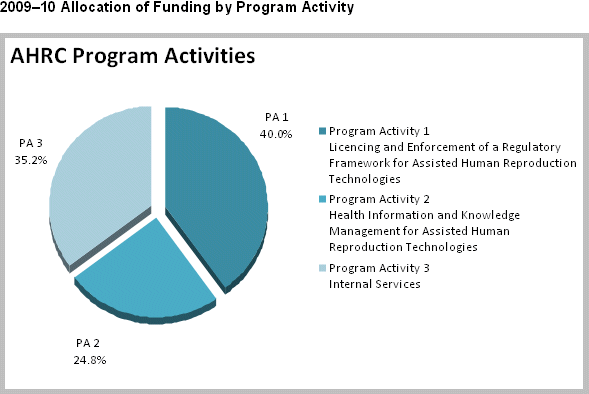
| Licencing and Enforcement of a Regulatory Framework for Assisted Human Reproduction Technologies |
An effective and efficient licencing and inspection framework. A well-informed and engaged stakeholder community. |
4.2 | 4.2 | 4.2 | Healthy Canadians |
| Health Information and Knowledge Management for Assisted Human Reproduction Technologies |
A Personal Health Information Registry that complements an eventual AHR surveillance network. Policy makers, health professionals, patients, children born of AHR procedures, researchers and the Canadian public have access to information regarding assisted human reproduction. |
2.6 | 2.6 | 2.6 | Healthy Canadians |
| Internal Services | Quality delivery of service in a cost-effective and timely manner supporting Program Activities 1 and 2. | 3.7 | 3.7 | 3.7 | Healthy Canadians |
| Total Planned Spending | 10.5 | 10.5 | 10.5 | ||
Contribution of Priorities to Strategic Outcomes
| Operational Priorities | Type | Links to Strategic Outcome | Description |
|---|---|---|---|
| To contribute to the development of AHR regulations by Health Canada | Previously committed to | SO 1 | Health Canada is in the process of developing regulations under the AHR Act that AHRC will administer. The Agency is working to actively contribute to Health Canada’s regulatory development process, which will establish the regulatory framework for the delivery of the AHRC mandate. |
| To increase the awareness of Canadians and health professionals about AHR and the Agency’s role | Previously committed to | SO 1 | Through its outreach, education and communication activities, Assisted Human Reproduction Canada (AHRC) will build awareness of its mandate and understanding of the AHR Act and regulations. Through its Web site and written materials, AHRC will inform Canadians about AHR. |
| To create the capacity in AHRC to implement the regulations (once developed) | Previously committed to | SO 1 | The Agency is putting in place the systems and processes required to implement the regulations currently under development by Health Canada, particularly those related to licencing, inspections, and the Personal Health Information Registry. |
| Management Priorities | Type | Links to Strategic Outcome(s) | Description |
|---|---|---|---|
| Internal Management | New | SO 1 | The Agency will develop and integrate its planning and reporting instruments, including Board of Directors strategic planning, integrated Agency-level business, and human resources planning and reporting. Planning and reporting will be aligned with the Program Activity Architecture, with increased emphasis on performance measurement and data collection. |
| Human Resources | New | SO 1 | As a new agency, AHRC is establishing its capacity in the specific areas needed to administer the regulations, as well as the processes and systems to support that role. Core capacity has been established, but as the Agency evolves, it will need to attract additional qualified resources in specialized areas such as privacy, systems maintenance, inspection, licencing, communications, etc. |
| Risk Management | New | SO 1 | The Agency is building an interim corporate risk management framework to manage its key risks. This will include the development and implementation of an ongoing process for monitoring, reporting and updating the risk management framework. This framework will be reviewed and updated once the regulations come into force. |
Risk Analysis
AHRC has been actively monitoring and managing its risks since the Agency was established. It recently launched a systematic review of its risks to fully document its plans to manage those risks, and to integrate a risk monitoring and reporting process into its planning cycle. This will serve as the Agency’s interim corporate risk management framework, which will be updated and revised as the full regulatory framework is implemented.
Few fields are changing faster than assisted human reproduction. Each technological innovation brings with it health, ethical, social and economic implications. AHRC is putting in place the resources and mechanisms to enable it to keep abreast of a rapidly changing environment, in terms of both science and international developments. One such mechanism is the recently established Science Advisory Panel, which brings together recognized experts in the field.
As a new Agency, AHRC is challenged to put in place the infrastructure (i.e., policies, procedures and systems) to effectively and efficiently manage its operations, as well as the specialized systems that will be required to implement the regulatory process once the regulations come into force. The Agency is implementing an information management framework that will ensure the continuity and safeguarding of corporate information, currently and when operations move to the Agency headquarters in Vancouver. The important systems that will be required to administer the regulations and Personal Health Information Registry are being developed on a task-based and modular basis, using a best practices approach to ensure alignment with the regulations being developed by Health Canada. Specialized resources are being retained to ensure the appropriate analysis and safeguarding of the personal health information the Agency will eventually be collecting.
From the outset, AHRC has recognized the importance of collaborating with various stakeholder groups to deliver on its mandate. The Agency is developing a comprehensive stakeholder outreach strategy that will build on its successful efforts to identify and ensure appropriate engagement of stakeholders. Fostering public and stakeholder awareness and understanding will be key to successfully meeting Agency objectives.
Resource planning is a challenge for the Agency, as it must take into account the completion of the establishment of the organization, the anticipated timing of the regulations coming into force, and plans to move operations to Vancouver. Key positions have been staffed with indeterminate employees, and integrated business and human resource plans are reviewed and updated on a regular basis.
Expenditure Profile
For the 2009–10 fiscal year, Assisted Human Reproduction Canada plans to spend $10.5 million to meet the expected results of its program activities and contribute to its strategic outcome.
The figure below illustrates Assisted Human Reproduction Canada’s spending trend from 2006–07 to 2011–12.
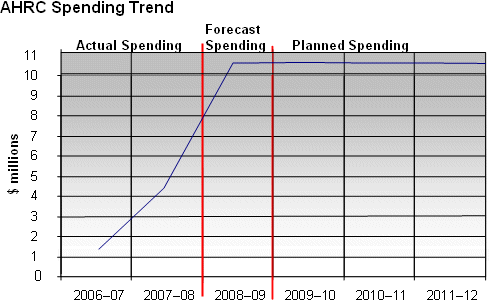
For the 2006–07 to 2008–09 periods, the total spending includes all Parliamentary appropriations (Main Estimates, Supplementary Estimates and Treasury Board Vote 15) as well as carry forward adjustments. For the 2009–10 to 2011–12 periods, the total spending corresponds to the planned spending. Supplementary funding and carry forward adjustments are unknown at this point and are therefore not reflected.
AHRC began operations in February 2007, and spending over its first few months of existence (in 2006–07) totalled $134,000.
From 2007–08 to 2008–09, AHRC’s spending has increased as the Agency has been fit up, staff have been hired and the infrastructure necessary to fulfill its mandate has been put in place. In 2009-10 and looking forward, the Agency will continue to monitor and enforce compliance with the AHR legislative and regulatory framework as it builds its capacity to protect and promote the health and safety of donors, patients and offspring born of assisted human reproduction technologies. Accordingly, emphasis will continue to be on resourcing and preparing systems for the regulations, once they come into force. At that time, Agency spending will become more centered on operations in delivering its regulatory mandate.
The figure below displays the allocation of AHRC’s funding by program activity for 2009-10.
Voted and Statutory Items
This table illustrates the way in which Parliament approved AHRC resources, and shows the changes in resources derived from supplementary estimates and other authorities, as well as how funds were spent.
| Vote # or Statutory Item (S) | Truncated Vote or Statutory Wording | 2008-09 Main Estimates ($ millions) |
2009-10 Main Estimates ($ millions) |
|---|---|---|---|
| 15 | Program expenditures | 11.8 | 9.9 |
| (S) | Contributions to employee benefit plans | 0.6 | 0.6 |
| Total | 12.4* | 10.5 | |
*Note: This figure is higher than in 2009–10 because it includes reprofiled funds from previous years.
