Common menu bar links
Breadcrumb Trail
ARCHIVED - Hazardous Materials Information Review Commission Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
President and Chief Executive Officer's Message
As the President and Chief Executive Officer of the Hazardous Materials Information Review Commission (HMIRC), I am pleased to submit to Parliament, and to Canadians, the Commission's 2009-10 Report on Plans and Priorities. The attached report reflects the Commission's commitment to be an important health and safety advocate for Canadian workers and a strategic partner to industry, helping to safeguard trade secrets and support market competitiveness.
The past year was one of change and transition. We worked diligently with our clients and stakeholders to streamline Commission operations through statutory and regulatory amendments and administrative process changes. Completion of this ten-year renewal program was marked by the enactment of legislation and the coming into force of important regulatory changes on October 1, 2008. Operational efficiency continues to be our number one priority as we begin the implementation phase of our journey.
However, capacity is the single most chronic issue crippling the Commission and jeopardizing its ability to deliver on its statutory mandate. This coupled with a substantial increase in the volume and complexity of claims for exemption from trade secret disclosure has resulted in a claim processing backlog of approximately two years. The backlog of claims causes a considerable delay in the provision of corrected health and safety information to workers as a result of the scientific review conducted by the Commission's staff.
When examined in the context of violation rates as reported by this agency - 95% non-compliance with 60% of errors being false or unstated health and safety information related to significant areas such as toxicological properties, hazardous ingredient disclosure and first aid measures - clearly, this represents a risk that must be met with corrective action.
Risk mitigation measures have been put in place, including the implementation of a pilot program aimed at educating clients up front and encouraging them to voluntarily correct Material Safety Data Sheets (MSDSs). Equally important is the Commission's claim prioritization scheme which targets high hazard claims for priority treatment. Notwithstanding these initiatives, the backlog continues to persist.
In order to address this issue, HMIRC is committed, through this report, to eliminating the backlog over a three-year period (2008 - 2011) through an increase in resources devoted to claim processing and core mandate support.
Based on the specific skills-set and training required for HMIRC's scientific review process, and the current shortage of qualified biologists/chemists, a period of three years was determined to be the timeframe over which resources could be used most efficiently in reducing our current backlog of claims. A Human Resource Strategy and Performance Measurement Plan have been developed to ensure that the challenges of functional area shortages are overcome and performance objectives are achieved.
This agency also participated in Phase 5 of the Management Accountability Framework (MAF) assessment exercise and was recognized for having good structures in place for a small agency. This includes the Commission's performance framework and managing organizational change. As a small agency, with limited administrative resources, the MAF confirmed our view that the Commission lacks the resources to develop a structured risk management framework and an information management system. These two areas were highlighted in our Program Integrity Business Case for supplementary funding.
The Commission occupies a unique place as Canada's centre of expertise for the review of claims for the protection of confidential business information and evaluation of MSDSs for compliance with federal/provincial/territorial OSH legislation. We need to plan for our role in the future as Canada implements the Global Harmonized System for the Classification and Labelling of Chemicals (GHS). The Commission will continue to monitor implementation the GHS in Canada to ensure that any changes needed to our operational practices with respect to GHS implementation are made.
While the year ahead will continue to focus on the implementation of many important operational initiatives, we also are committed to informing and defining this agency's vision for the next 3 to 5 years.
The Commission's vision is to serve the stakeholders we were created to protect and support - to continue to better serve Canadians and invest in efficiencies and streamlining measures - by engaging claimants and stakeholders, and partnering with organizations to share best practices and lessons learned.
HMIRC has built a relationship with its Council of Governors, representing stakeholders, based on trust, respect and a shared vision. Our approach is simple and straightforward - Canadian taxpayers' interests are best served by considering and balancing the needs of workers and industry alike, and the solution is one that protects both.
I look forward to collaborating with our Council of Governors as we move into the planning year which will, no doubt, present a continuum of challenges and opportunities. I am grateful for the dedication of the Commission's staff and continue to be impressed by the quality and commitment of the people I have the privilege to work with in this agency.
Sharon A. Watts
President and Chief Executive Officer
Section I: Departmental Overview
Health Portfolio Overview
The Minister of Health, through the work of the Health Portfolio, is responsible for maintaining and improving the health of Canadians. In addition to the Hazardous Materials Information Review Commission (HMIRC), the Portfolio consists of Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Patented Medicine Prices Review Board and Assisted Human Reproduction Canada. Each member of the Portfolio prepares its own Report on Plans and Priorities.
The Health Portfolio consists of approximately 12,000 full-time equivalent employees, with an annual budget of over $3.8 billion.
HMIRC'S Raison d'être
| HMIRC provides a mechanism for protecting the trade secrets of those companies that manufacture, supply and/or use hazardous materials, and it provides accurate information about the intrinsic health and safety hazards to Canadian employees who work with such materials. |
Responsibilities
The Commission enables chemical companies to protect their trade secrets, and while doing so ensures that accurate health and safety information about hazardous chemicals is available to workers in order to reduce workplace-related illness and injury. The Commission's activities are key components of the Workplace Hazardous Materials Information System (WHMIS), which was created in the late 1980s through a consensus of labour, industry and government. The success of WHMIS depends on cooperation among all these partners. All three groups play an integral part in ensuring that the information workers need to know about hazardous chemical products, is available.
WHMIS requires that manufacturers and suppliers provide employers with information on the hazards of materials produced, sold, or used in Canadian workplaces. The employers, in turn, provide that information to employees through product labels, worker education programs, and material safety data sheets (MSDSs). A product's MSDS must fully disclose all hazardous ingredients in the product, its toxicological properties, any safety precautions workers need to take when using the product and treatment required in the case of exposure.
When a supplier introduces a new or improved product and wants to protect the identity of one or more of the ingredients or the concentration, the company applies to HMIRC for an exemption from the requirement to list all hazardous ingredients on the product's MSDS. Once the application is complete, HMIRC registers the claim and the product can be made available in the marketplace. The Commission then issues a decision on the validity of the claim and, to protect worker safety, verifies the compliance of the MSDS and, in some cases, the label with the Hazardous Products Act and Controlled Products Regulations, and with provincial and territorial occupational health and safety acts. The Commission offers voluntary compliance undertakings which if not accepted lead to the issuance of orders for any changes that are necessary to the MSDS or product labels.
The Hazardous Materials Information Review Act and associated regulations, as applied by HMIRC, provide a mechanism to balance the competing rights of business and workers. The Commission is an independent agency that plays a quasi-judicial role in delivering WHMIS and representing the interests of federal, provincial and territorial governments, as well as workers, employers and members of the chemical industry.
Role of the Commission
Mandate
The Hazardous Materials Information Review Act mandates the Commission to:
- Register claims for trade secret exemptions and issue registry numbers.
- Adjudicate and issue decisions on the validity of claims for exemption on the basis of prescribed regulatory criteria.
- Make decisions on the compliance of Material Safety Data Sheets (MSDSs) and labels with Workplace Hazardous Materials Information System (WHMIS) requirements.
- Convene independent boards with representatives of labour, suppliers or employers to hear appeals from claimants or affected parties on decisions, undertakings and orders.
If a supplier or employer wants to withhold information that they believe to be a trade secret, they must file a claim with the Commission for exemption from its WHMIS obligations to disclose this information. The screening officers review these claims against the applicable federal, provincial or territorial regulations, and rule on the claims' validity. This process involves communication between evaluators, screening officers and claimants, to ensure transparency.
The scientific evaluators play a key health and safety role in the claim review process. They review the MSDSs and labels associated with a claim for exemption to ensure the health and safety information is complete and accurate in accordance with WHMIS requirements, based on the Hazardous Products Act, the Canada Labour Code, and the Controlled Products Regulations, and provincial and territorial occupational health and safety legislation. This helps ensure that workers are informed of the hazards of exposure to chemicals found in products associated with claims for exemptions. When evaluators identify missing or incorrect information, they advise the screening officers who issue a decision statement along with a voluntary compliance undertaking which sets out the measures the claimant must take to have the MSDS conform to WHMIS requirements. To indicate acceptance of the compliance undertaking claimants signs and returns the undertaking and the corrected MSDS to the Commission. If claimants do not accept the undertaking, the screening officers order the claimant to make the necessary changes through formal orders. Formal orders require claimants to make the necessary changes and to submit the corrected MSDS within 75 calendar days of the publication of the orders in the Canada Gazette.
The Commission also convenes independent boards to hear appeals from claimants or affected parties challenging decisions, undertakings and/or orders.
In addition, HMIRC responds to requests from federal, provincial and territorial government health and safety officials for information about claims for exemption to help these officials administer and enforce their WHMIS obligations.
Values and Operating Principles
The Commission recognizes that continuous improvement is critical in order to remain relevant and to provide effective and efficient performance and service quality. HMIRC has identified the values and operating principles that foster continuous improvement in its operations.
FAIRNESS - in its ability to provide services and to perform statutory functions.
TIMELINESS - in its ability to provide services within established and reasonable time frames.
ACCESSIBILITY and TRANSPARENCY - in its ability to provide information and services simply and clearly and with policies and procedures that are understandable to everyone.
ACCOUNTABILITY - in its ability to propose legislative approaches only when they meet rigorous cost-benefit analysis and to be accountable for programs and the impact of decisions, while providing services in a manner that is cost-effective for everyone involved.
QUALITY and CONSISTENCY - in its ability to render accurate, relevant, dependable, understandable, predictable and error-free decisions, while ensuring consistent, firm enforcement of the regulations.
COMPETENCY and RESPECT - in its ability to provide services based on a high level of skill, knowledge, scientific and technical competence, and to demonstrate respect and professionalism to everyone who comes into contact with the Commission.
SECURITY and CONFIDENTIALITY - in its ability to store and handle the trade secrets of its claimants.
Strategic Outcome and Program Activity Architecture (PAA)
Canada places the health of its population high on the list of key priorities. Canada's public health system exists to safeguard and improve the health of Canadians. The responsibility for public health is spread across federal, provincial and territorial governments. An essential part of any occupational health and safety program is ensuring that those employed in workplaces where hazardous materials are used have the information they need to use those materials without risk of injury and without short or long-term health threats. In this respect, an important part of the Commission's mandate is the scientific review of the completeness and accuracy of the health and safety information supplied to employers and workers using hazardous products. The Commission's work supports improved occupational health and safety for Canadian workers, a key element to achieving a healthy Canadian population.
The Commission also provides a mechanism for protecting the trade secrets of those companies which manufacture, supply and/or use hazardous materials. This part of the balancing equation supports the Government of Canada's strategic outcome of a fair and secure marketplace, which is directed at ensuring that the marketplace continues to foster competitive conditions to attract investment, encourage innovation, and protect the public interest. The Commission's mandate protects bona fide trade secrets and allows the marketplace to function competitively while at the same time, the Commission protects the public interest by ensuring that workers required to use hazardous materials have the information they need to use those materials safely.
Planning Summary
| 2009–20101 | 2010–2011 | 2011–2012 |
|---|---|---|
| 5,555 | 5,539 | 4,367 |
| 2009–2010 | 2010–2011 | 2011–2012 |
|---|---|---|
| 54 | 54 | 42 |
Summary Table:
| Strategic Outcome 1: | |||||
|---|---|---|---|---|---|
| Trade secret exemptions are provided in a way that balances the right of industry to protect their confidential business information with the right of workers to receive accurate information concerning the health and safety hazards posed by workplace chemicals in the workplace. | |||||
| Performance Indicators | Targets | ||||
| Number of appeals as a percentage of total claims processed. |
Zero | ||||
| Planned Spending (000's) | |||||
| Program Activity2 |
Forecast Spending 2008-093 |
2009–2010 | 2010–2011 | 2011–2012 | Alignment to Government of Canada Outcomes |
| Claims Exemption Process |
5,027 | 5,906 | 5,891 | 4,647 | Healthy Canadians |
| Total Planned Spending | 5,906 | 5,891 | 4,647 | ||
1 Through the 2009-10 ARLU, the Commission received supplementary funding of approximately $2.0 M for fiscal years 2009-10 and 2010-11. This amount was reduced to $850 K for fiscal years 2011-12 and beyond.
2 For program activity descriptions, please access the Main Estimates online at http://www.tbs-sct.gc.ca/est-pre/20082009/me-bd/pub/me-256_e.asp .
3 In fiscal year 2008-09, the Commission received a transfer of $1.7 M from Health Canada through the Supplementary Estimates A to address its backlog.
Contribution of Priorities to Strategic Outcome
Strategic Outcome - 1 Trade secret exemptions are provided in a way that balances the right of industry to protect their confidential business information with the right of workers to receive accurate information concerning the health and safety hazards posed by workplace chemicals in the workplace.
| Operational Priorities | Type | Links to Strategic Outcome | Description |
|---|---|---|---|
| Efficient Client Service Delivery | Ongoing | Strategic Outcome - 1 Program Activity - 1 |
To improve the health and safety of Canadian workers through service improvements to our clients and stakeholders, the Commission will continue the implementation of its plan to reduce/eliminate the 2 year backlog of claims to be processed. To increase the efficiency of the claims review process the Commission is developing an integrated data management system to create an electronic access to all information relevant to the assessment of claims; the database will be operational in 2009-10. The Commission will build on this by exploring the development of additional electronic tools to improve the review of MSDSs. Finally, the Commission will continue to implement the process changes and streamlining measures permitted through the amendments to the Hazardous Materials Information Review Act and Regulations. |
| Modernized Legislation | Ongoing | Strategic Outcome - 1 Program Activity - 1 |
As the unique centre of expertise for the Canadian trade secret mechanism within WHMIS, the Commission will monitor and contribute to international or domestic discussions concerning the protection of confidential business information. As well, the Commission will continue to monitor Canada's implementation of the requirements for the Globally Harmonized System of
Classification and Labelling of Chemicals to ensure that its operational practices are amended as required in order to be compliant with the new standards. When WHMIS was initially implemented in 1988, certain categories of products were exempted ("excluded") from the WHMIS supplier label and MSDS requirements of the Hazardous Products Act (HPA). The HPA exclusions are mirrored in the laws which set out WHMIS employer requirements established by each federal, provincial and territorial agencies responsible for occupational health and safety in Canada. The WHMIS exclusions may be re-examined in conjunction with the transition to the Globally Harmonized System (GHS). The Commission will monitor and participate in discussions on the exclusions examination to ensure that its operational practices are amended, as required by any changes. In addition, in the context of the proposed Food and Consumer Safety Action Plan, the federal government may once again consider amendments to the Hazardous Products Act. The Commission will also monitor and participate in these discussions to ensure its operational practices are amended, if necessary. |
| Management Priorities | Type | Links to Strategic Outcome | Description |
|---|---|---|---|
| Stakeholder Liaison and Partnerships | New | Strategic Outcome - 1 Program Activity - 1 |
The Commission is recognized as a centre of scientific expertise with respect to the evaluation of information that should be contained on MSDS and product labelling of hazardous chemicals within WHMIS. Through outreach and educational activities, such as regular client workshops, the Commission will share its expertise and build awareness of its important mandate within WHMIS. Partnerships with organizations with similar mandates will be pursued to share best practices and lessons learned. The Commission's Website will be leveraged to improve stakeholder liaison. At the end of 2008-2009, the Commission's Website featured a number of new electronic user tools aimed to support the legislative and regulatory amendments that introduced streamlining improvements to the claims review process. In 2009-2010, the Commission will continue to improve the effectiveness of its website as a primary communication and outreach tool for its claimants and stakeholders. |
| Management Excellence | Ongoing | Strategic Outcome - 1 Program Activity - 1 |
The Commission, as a small agency with a unique mandate, is continuously challenged in the area of recruitment and retention of qualified personnel. Strategies to address this challenge will include increased use of pre-qualified pools within the Health Portfolio and public service in general. As well, the Commission will focus on the development of internal resources
to meet current and future challenges through cross-training and creation of developmental assignments within the Commission. The Commission participated in Phase V of the 2007-08 Management Accountability Framework. A number of key areas for improvement were identified, along with many areas of strengths. An important priority for 2009-10 will be the establishment of a more formalized risk management framework. Another important priority for the planning year will be improving the effectiveness of the Commission's information management system through development and implementation of an electronic file classification system expected to be rolled out to all staff in 2009-10 along with guidelines and procedures for its use. |
Risk Analysis
The Commission's small size presents operational challenges. In 2007-08, the Commission operated with 35 FTEs and a budget of $3.5 million. About 85% of the Commission's budget is required for salaries; the remaining 15% is designated for non-discretionary or statutory program spending, leaving little or no flexibility for discretionary spending. Consequently, the Commission must carefully balance its human resources and discretionary spending between operational requirements and strategic initiatives. These can be both internally driven and externally mandated.
HMIRC is faced with challenges to meet its statutory mandate through a significant increase in both the number and the complexity of claims for exemption from trade secret disclosure. This, coupled with a chronic shortage of qualified scientific personnel and insufficient resources in supporting areas, has resulted in a claim processing backlog of approximately two years.
Despite the implementation of risk mitigation strategies, namely the claims prioritization scheme which targets high hazard claims for priority treatment, and the MSDS voluntary compliance program to increase the efficiency of MSDS corrections, the backlog of claims for processing persisted. As a result the Commission developed a Business Case for additional resources and received funding for this purpose.
Externally, HMIRC faces ongoing pressure to implement numerous government-wide initiatives, such as the Treasury Board Policy Suite Renewal and Management Accountability Framework (MAF), and the Federal Accountability Act, which are aimed at improving service and accountability to the Canadian public.
In addition, in the context of the proposed Food and Consumer Safety Action Plan, the federal government may once again consider amendments to the Hazardous Products Act (HPA). The Commission occupies a unique place as Canada's only agency that manages the registration of claims by industry for protection of confidential business information of hazardous workplace chemicals under WHMIS. Therefore, any changes to national or international legislation that impacts the protection of confidential business information has the potential to impact the work of the Commission.
Furthermore, Canada has publicly declared a commitment to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), in that the implementation of GHS and adjustments to the current scheme are on the horizon. However, the extent of the adjustments resulting from GHS and the impact of these adjustments on the work of the Commission is not fully known at this time.
Internally, HMIRC's dependence on highly qualified scientific personnel for its claims review process and the shortage of personnel in this group renders recruitment a risk. The fact that HMIRC is a small organization with few opportunities for career advancement makes retention of qualified personnel a challenge. These factors combined make recruitment and retention a challenge to realizing the work of the Commission.
According to demographic studies, the number of public servants eligible for retirement is steadily increasing. The impact on the workforce and corporate memory of government departments and agencies will be especially prevalent in the next three to five years. Addressing the turnover of staff through succession planning and knowledge transfer is key to HMIRC being able to fulfill its mandate.
Expenditure Profile
In 2007-08, HMIRC developed a plan to eliminate its backlog of claims for exemption over a three year period (2008-2011) and to prevent it from recurring. However, due to its very small A-Base, which in fiscal year 2007-08 totalled $3.5 M, the Commission sought supplementary funding for each of the three years, as well as ongoing funding, for 2011-12 and beyond, to implement the plan.
In January 2008, Health Canada (HC) and HMIRC, in consultation with the Treasury Board Secretariat, and with concurrence of the Minister of Health, agreed that Health Canada would transfer $1.7 M to HMIRC to begin the work required to address the backlog through the 2008-09 Supplementary Estimates A. The Annual Reference Level Update was identified as the appropriate mechanism for transferring supplementary funding of $2 M each year for 2009-2010 and 2010-2011, as well as $850K for 2011-2012 and ongoing.
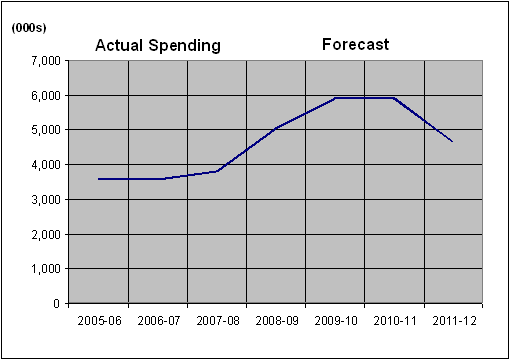
The figure below illustrates HMIRC's planned spending from 2005-06 to 2011-12.
Departmental Spending Trend
Voted and Statutory Items
| Vote # or StatutoryItem (S) | Truncated Vote or Statutory Wording | 2008–09 Main Estimates |
2009–104 Main Estimates |
|---|---|---|---|
| 30 | Program expenditures | 3,097 | 4,855 |
| (S) | Contributions to employee benefit plans | 468 | 700 |
| TOTAL | 3,565 | 5,555 | |
4 Through the 2009-10 ARLU, the Commission received supplementary funding of approximately $2.0 M for fiscal years 2009-10 and 2010-11. This amount was reduced to $850 K for fiscal years 2011-12 and beyond.
