Common menu bar links
Breadcrumb Trail
ARCHIVED - Patented Medicine Prices Review Board - Report
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Chairperson’s Message
I am pleased to present the 2012-13 Report on Plans and Priorities for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is committed to fairness and transparency in carrying out its mandate. In 2011, we focussed on assessing our direction in light of ongoing shifts in the health care environment. We are seeing important changes, domestically and internationally, as distribution practices evolve, sales models change, patentees introduce different types of benefit programs, and new types of drugs continue to reach the market.
The PMPRB’s objective to ensure that Canadians do not pay excessive prices for patented medicines is an important one which contributes to the government-wide goal of healthy Canadians. It impacts on public and private payers and on cash-paying customers. The decision issued by the Supreme Court of Canada on January 20, 2011, upholding key aspects of the Board’s jurisdiction, provided an important clarification and affirmation of the PMPRB’s consumer protection role. To that end, we have modified our engagement policy with stakeholders and enhanced our outreach to patentees. Our Monitoring and Evaluation Plan for the Major Changes in the Guidelines provides opportunities for a continued dialogue with stakeholders and allows for timely adjustments to our Guidelines. It is our intention that the Guidelines be responsive to changes in the drug distribution and pricing environment, in an appropriate timeframe.
For the coming year, our priorities are to enhance compliance by examining alternate dispute resolution models, to explore possible ways of decreasing regulatory burden for patentees and to make the most effective use of our resources while remaining open and transparent. Enhancements to our engagement with stakeholders will also go a long way to meeting our long-standing commitment to a regulatory regime that is relevant, responsive and appropriate.
We have not lost track of our reporting role. Through the National Prescription Drug Utilization Information System (NPDUIS), we continue our partnership with the Canadian Institute for Health Information, Health Canada and the provinces and territories. We have published several reports in the past year and have initiated a number of studies which will provide policy makers and drug plan managers with information and insights on trends in prices, utilization and costs.
As Chair of the PMPRB it is my objective to ensure that our framework continues to have a positive impact for consumers while recognizing the value that innovative medicines offer to patients. The PMPRB remains committed to effectively meeting challenges, serving Canadians, and contributing to the health care system.
Mary Catherine Lindberg
Chairperson
Section I: Organizational Overview
Raison d’être
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi‑judicial body created by Parliament in 1987. Its mandate is two‑fold:
- Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting – to report on pharmaceutical trends of all medicines and on R&D spending by pharmaceutical patentees.
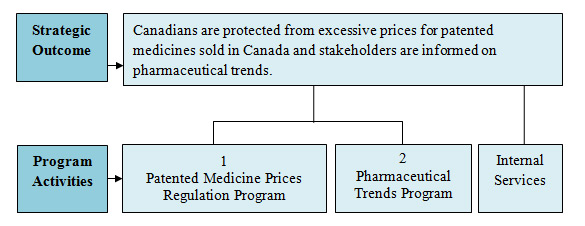
In carrying out its mandate, the PMPRB endeavours to ensure that Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
Responsibilities
The PMPRB was created as a result of revisions to the Patent Act (Act) in 1987 (Bill C-22). The Act was further amended in 1993 (Bill C-91). The revisions were intended to balance the extension of patent protection with the need to protect consumers from possible excessive patented drug prices.
Regulatory Role
The PMPRB is responsible for regulating the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, to ensure that they are not excessive. The PMPRB regulates the price of each patented medicine (each strength of each individual, final dosage form of a medicine). This is normally the level at which Health Canada assigns a Drug Identification Number (DIN) as part of the Notice of Compliance process. However, the Board’s mandate also includes medicines available under the Special Access Programme; medicines available through a Clinical Trial Application; and Investigational New Drug Products. Over-the-counter (OTC) patented medicines and patented medicines for veterinary use are also under the Board’s jurisdiction but are dealt with on a complaints basis.
The Federal Court of Appeal articulated the legal requirement as to when a patent will “pertain” to the medicine. In this regard, the Court established the “merest slender thread” requirement, which is wide in scope. The Board’s jurisdiction is not limited to medicines for which the patent is on the active ingredient. Rather, the Board’s jurisdiction also covers medicines for which the patents relate, but are not limited, to the processes of manufacture, the delivery system, dosage form, indication/use, and/or any formulation. Patented medicines are not limited to brand name medicines. A number of generic companies fall under the Board’s jurisdiction by virtue of being licensees (i.e. authorized to sell the same medicine as the brand company is selling) or due to their own patents (e.g. related to processes of manufacture).
The PMPRB has no authority to regulate the prices of non-patented medicines and does not have jurisdiction over prices subsequently charged by wholesalers or retailers or over pharmacists’ professional fees. In addition, matters such as whether medicines are reimbursed by public or private drug plans, distribution channels and prescribing are outside the purview of the PMPRB.
Under the Act, patentees are required to inform the PMPRB of their intention to sell a new patented medicine. Upon the sale of such a patented medicine, as per the Patented Medicines Regulations, patentees are required to file price and sales information for the first day’s sales and, thereafter, twice a year for six month periods (January to June and July to December) for each strength of each dosage form of each patented medicine sold in Canada for price review/regulation purposes, for the duration of the patent(s).
Although patentees are not required to obtain the PMPRB’s approval of the price of a patented medicine before it is sold, they are required to comply with the Act to ensure that prices of patented medicines sold in Canada are not excessive. Advisory assistance is, however, provided to those patentees who wish to consult with Board Staff prior to introduction of a patented medicine. If a patented medicine is sold before the patent issues, the PMPRB will review the price of the medicine as of date of first sale, as long as this is after the date on which the patent application was laid open for public inspection.
In the event that the Board finds, after a public hearing, that a price is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting Role
The PMPRB reports annually to Parliament through the Minister of Health. The Annual Report, which covers each calendar year, includes a review of the PMPRB´s major activities, analyses of the prices of patented medicines and of the price trends of all medicines, and reports on the R&D expenditures as reported by patent-holding drug manufacturers. In addition, the PMPRB reports through its quarterly NEWSletter and various studies.
Pursuant to an agreement by Federal/Provincial/Territorial Ministers of Health, and at the request of the federal Minister of Health, the PMPRB has partnered with the Canadian Institute for Health Information (CIHI) through the National Prescription Drug Utilization Information System (NPDUIS) initiative. Under this initiative, the PMPRB provides critical analyses of drug prices, utilization, and cost trends in Canada to support drug plan policy decision making for participating federal, provincial, and territorial governments. In 2005, the federal Minister of Health, on behalf of F/P/T Ministers of Health, directed the PMPRB to monitor and report on non-patented prescription drug prices. Since 2008, this work has been conducted under the umbrella of the NPDUIS initiative.
Strategic Outcome(s) and Program Activity Architecture (PAA)
Organizational Priorities
| Priority | Type1 | Strategic Outcome(s) and/or Program Activity(ies) 2 |
|---|---|---|
| Enhance compliance with the Board’s Guidelines | New | Links to Program Activity 1 |
| Description | ||
|
Why is it a priority? In June 2009, after extensive consultations with its key stakeholders the Board revised its Guidelines and a new Compendium of Policies, Guidelines and Procedures came into effect on January 1, 2010. The revised Guidelines are intended to assist pharmaceutical patentees in establishing non-excessive prices by providing transparent and predictable information on how the price review will be undertaken. Enhanced compliance with the Guidelines will help ensure that Canadians are protected from excessive prices for patented medicines sold in Canada. The PMPRB also developed a Monitoring and Evaluation Plan for the Major Changes in the Guidelines and has completed in 2011-12, its first annual assessment of the application and impact of the changes to the Guidelines. While it may take several reporting periods before any discernible trends become evident, the Board will monitor emerging issues to identify the need for any future amendments to the Guidelines or the monitoring and evaluation plan. Plans for meeting the priority
|
||
| Priority | Type | Strategic Outcome(s) and/or Program Activity(ies) |
|---|---|---|
| Decrease regulatory burden and make effective use of Board Staff resources | New | Links to Program Activity 1 & 2 |
| Description | ||
|
Why is it a priority? In January 2011 the Prime Minister launched the Red Tape Reduction Commission, as part of the Economic Action Plan. The Commission was asked to identify irritants to businesses that have clear detrimental effects on growth, competitiveness and innovation, and to recommend ways to address those irritants and reduce the compliance burden on a lasting basis without compromising the environment or the health and safety of Canadians. Irritants identified by the pharmaceutical industry were ultimately determined to be outside the scope of this initiative, and did not figure in the Commission’s report. Nonetheless, the PMPRB hopes to support the spirit of this initiative by examining its price review process to identify possible ways to reduce the regulatory burden on patentees without adversely affecting its mandate to protect consumers. In addition, as a result of the Budget 2010 Cost Containment Measures, which froze departmental operating budgets at their 2010-11 levels, the PMPRB identified certain positions that would remain vacant. In doing so, certain operational efficiencies were adopted, and others may be necessary, to ensure the most effective use of resources. Plans for meeting the priority
|
||
| Priority | Type | Strategic Outcome(s) and/or Program Activity(ies) |
|---|---|---|
| Transparency and communications | New | Links to Program Activity 1 & 2 |
| Description | ||
|
Why is it a priority? This initiative is aimed at making all stakeholders more aware of the services and information that the PMPRB offers. In order to effectively fulfill its mandate, the PMPRB must ensure that stakeholders are both informed and engaged. Stakeholders must understand and appreciate the PMPRB’s role as a contributor to the health care system. Plans for meeting the priority
|
||
Risk Analysis
The PMPRB is responsible for ensuring that the prices (“factory gate prices”) patentees charge for patented medicines sold in Canada for human or veterinary use are not excessive. Like many regulators, the PMPRB promotes and relies on voluntary compliance as a means to effectively use resources and to control its costs and the costs for patentees. In addition, voluntary compliance often represents the timeliest way to ensure that Canadian consumers and payers benefit from the non-excessive pricing of patented medicines. The PMPRB has been largely able to carry out its mandate with limited recourse to public hearings due, at least in part, to the rate of compliance with the Board’s Excessive Price Guidelines (Guidelines) and the use of Voluntary Compliance Undertakings (VCUs) incorporated into its voluntary compliance policy.
For approximately five years, the Board undertook a comprehensive review of its Guidelines, and in January 2010, its revised Guidelines came into effect. The new Guidelines are intended to ensure the continuing effectiveness, fairness, transparency, predictability and relevance of the price review process in today’s current and evolving pharmaceutical environment. Two of the more significant changes were intended to address challenges respecting: a) incremental innovation and b) the practice of patentees of offering benefits to consumers and payers as part of their business and distribution models. Early indications are that these two elements have improved compliance and assisted in the effective management of the PMPRB’s resources. In response to challenges experienced in the operationalization of the new Guidelines, the Board was quick to clarify the interpretation and application of its Guidelines and to adopt approaches to facilitate efficient implementation of the new elements.
In 2011-12, the PMPRB approved a Guidelines Monitoring and Evaluation Plan (GMEP). Monitoring and evaluating the application and impact of the revised Guidelines will mitigate risks to the organization through early identification and resolution of Guidelines-related issues. Board Staff will be better able to address challenges for pharmaceutical patentees and other stakeholders as they arise.
The PMPRB’s capacity to carry out its regulatory mandate is focused on its ability to conduct timely price reviews, investigations and, when needed, hearings. By and large, patentees, comply with the Patent Act and the Board’s Guidelines by not charging excessive prices (roughly 90% compliance). In 2012-13, in an effort to maintain this level and to support even greater compliance, the PMPRB has identified two priorities: a) to consider alternate dispute resolution models; and, b) to explore means to reduce regulatory burden for patentees and to effectively use staff resources.
The pricing and reimbursement environment in Canada is one that evolves over time and which has changed significantly over the recent year. In addition, the sales of patented medicines and the nature and distribution of these medicines are also evolving into areas that are more complex and innovative. The challenge for the PMPRB is to remain effective in delivering its mandate in the face of a changing environment and to do so within the constraints of increasing fiscal prudence. The risk of failure and the consequence of failing at this task would have a negative effect on the pricing framework for patented medicines in Canada and the increased cost to payers would be significant.
Internationally, changes respecting the pricing and reimbursement of patented medicines are also being made. In the coming years, prices of patented medicines are expected to fall in Germany and the United Kingdom, in particular. Since they are included in the basket of countries used by the PMPRB for price review purposes, their policy and pricing changes will be monitored closely. In addition, the PMPRB will continue to monitor international trade negotiations which may include an intellectual property component (e.g. current negotiations between the European Union and Canada) for any potential impact they may have on the PMPRB’s legislative and regulatory framework. The Board’s priorities for 2012-13 may require adjustment should there be any significant domestic or international policy or legislative changes.
The PMPRB engaged external experts to conduct an evaluation of its programs in 2011-12. The results of the evaluation which are expected to be finalized in the spring of 2012 may impact program design and administration. In addition, the results of the Program Evaluation may require changes to the Guidelines. A Management Action Plan will be developed and adopted in 2012-13 and a tracking system will ensure implementation over time of elements of the plan.
The Board has identified the need to be responsive to the changing environment and continues, therefore, to focus on transparency and communications. For example, Board funds continue to be committed to holding face-to-face outreach sessions with patentees and to improving the accessibility and usefulness of material on the Board’s website. The Board continues to communicate regularly with patentees and other stakeholders through various means and fora. Through its Pharmaceutical Trends Program, the PMPRB provides analysis of pharmaceutical price trends and research and development spending by pharmaceutical patentees. It also provides critical analyses of price, utilization and cost trends for prescription drugs, and information on non-patented prescription drug prices. The challenge for the PMPRB is to ensure that its reports provide relevant information and are issued in a timely manner such that they will provide credible pharmaceutical trend information and will contribute to the information needs of a variety of policy decision-makers.
Workload is expected to remain relatively steady over the coming year, while the Board’s resources may be challenged due to a changing human resources profile, given retirement eligibility and changing workforce demographics. A Succession Plan has been adopted by PMPRB senior management which may address some of these challenges. The PMPRB also continues to employ an integrated human resources and business planning model.
Planning Summary
Financial Resources ($ thousands)
| 2012–13 | 2013–14 | 2014–15 |
|---|---|---|
| 11,832.4 | 11,832.4 | 11,832.4 |
Human Resources (FTEs)
| 2012–13 | 2013–14 | 2014–15 |
|---|---|---|
| 76.0 | 76.0 | 76.0 |
| Performance Indicators | Targets |
|---|---|
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations | Canada's prices on average are at or below the median of international prices |
| Program Activity | Forecast Spending 2011–12 | Planned Spending | Alignment to Government of Canada Outcomes | ||
|---|---|---|---|---|---|
| 2012–13 | 2013–14 | 2014–15 | |||
| PA 1: Patented Medicine Prices Regulation Program |
5,078.8 | 7,508.1 | 7,508.1 | 7,508.1 | Healthy Canadians |
| PA 2: Pharmaceutical Trends Program |
919.6 | 1,265.4 | 1,265.4 | 1,265.4 | Healthy Canadians |
| Total Planned Spending | 8,773.5 | 8,773.5 | 8,773.5 | ||
| Program Activity | Forecast Spending 2011–12 | Planned Spending | ||
|---|---|---|---|---|
| 2012–13 | 2013–14 | 2014–15 | ||
| Internal Services | 3,447.0 | 3,058.9 | 3,058.9 | 3,058.9 |
| Total Planned Spending | 11,832.4 | 11,832.4 | 11,832.4 | |
Expenditure Profile
Departmental Spending Trend
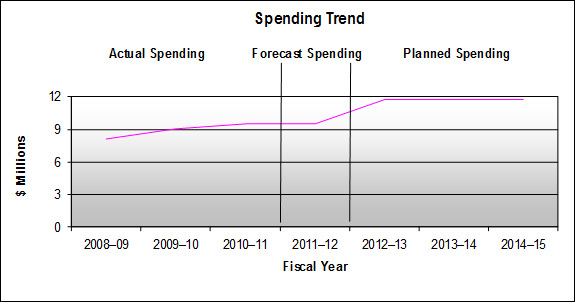
In 2008-09 Treasury Board granted the PMPRB permanent funding in the amount of $5.1 million including the employee benefit plan (EBP) and excluding Public Works and Government Services Canada (PWGSC) accommodation charges, in addition to its core A-base of $5.8 million, to enable the PMPRB to meet its workload pressures and continue ongoing initiatives related to the delivery of its mandate. Treasury Board also granted authority to increase reference levels in Vote 35 (Program expenditures) by $5.6 million for 2009-10, $6.2 million for 2010-11, and $5.8 million for 2011-12 and future years (including EBP and excluding PWGSC accommodation charges).
Included in the additional funding were $1.9 million in 2008-09, 2.2 million in 2009-10, and $2.8 million in 2010-11 and subsequent years, in the Special Purpose Allotment (SPA) reserved for the conduct of public hearings. This increased total ongoing funding for the SPA to $3.1 million. The SPA can only be used to cover the costs of public hearings such as external legal counsel and expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF).
As a result of difficulty hiring new staff and a limited number of hearing days, the PMPRB did not spend its entire budget in the period from 2008-09 to 2010-11. The PMPRB now has all of the positions it intends to fill staffed and it is anticipated that actual spending (excluding the SPA) will closely resemble funding provided.
With respect to the SPA, expenditures on services such as external legal counsel and expert witnesses are completely dependent on the number of hearings held and the length and complexity of the hearings, and are therefore very difficult to predict. Any SPA funds not required for hearings will be returned to the Consolidated Revenue Fund.
As a result of the Budget 2010 Cost Containment Measures, departmental operating budgets were frozen at their 2010-11 levels.
Estimates by Vote
For information on our organizational appropriations, please see the 2012–13 Main Estimates publication.
