Common menu bar links
Breadcrumb Trail
ARCHIVED - Patented Medicine Prices Review Board Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Chairperson's Message
I am pleased to present the 2010-2011 Report on Plans and Priorities for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act. Its mandate is two-fold: Regulatory — to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and Reporting — to report on pharmaceutical trends of all medicines and on R&D spending by pharmaceutical patentees.
The PMPRB plays an important role in the broader objective of improving the health of Canadians by protecting consumers and the health care system from excessive patented drug prices.
After extensive consultation with key stakeholders, as required by the Patent Act, new Excessive Price Guidelines (Guidelines) were released on June 9, 2009, for implementation on January 1, 2010. Monitoring and evaluation of the application and impact of the changes will be a key focus in 2010-2011 and beyond.
In recent years, the PMPRB has experienced a significant increase in the number of hearings to determine if the price of a patented drug product is excessive. While some hearings were completed in 2009-2010, a number are still ongoing, and new hearings remain a possibility.
The PMPRB will continue to enhance educational outreach to patentees and other stakeholders while it implements the revised Guidelines. It will initiate the establishment of processes to streamline price reviews and investigations. The Board will also review its rules governing the hearing process with a view to completing hearings in a more timely and efficient manner.
The PMPRB reports annually to Parliament, through the Minister of Health, on its activities, on pharmaceutical trends relating to all patented drug products, and on the R&D spending by pharmaceutical patentees. The PMPRB will continue to work in collaboration with the Canadian Institute for Health Information (CIHI) and participating federal/provincial/territorial drug plans to produce analyses and reports under the National Prescription Drug Utilization Information System (NPDUIS). Through critical analyses of price, utilization and cost trends, the PMPRB provides Canada's health system with comprehensive, timely and accurate information on prescription drug trends and cost drivers.
The PMPRB remains committed to predictability, fairness and transparency in the fulfilment of its regulatory and reporting responsibilities, to ongoing engagement of its key stakeholders, and to continual monitoring of developments within the larger pharmaceutical environment.
The original version was signed by
Brien G. Benoit, MD
Chairperson
Section I — Board Overview
Raison d'être and Responsibilities
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body created by Parliament as a result of revisions to the Patent Act (Act) in 1987 (Bill C-22). The Act was further amended in 1993 (Bill C-91). The revisions were intended to balance the extension of patent protection with the need to protect consumers from possible excessive patented drug prices.
The PMPRB has a dual role:
Regulatory — To ensure that prices charged by patentees for patented medicines sold in Canada are not excessive.
Reporting — To report on pharmaceutical trends of all medicines, and on R&D spending by pharmaceutical patentees.
Regulatory Role
The PMPRB is responsible for regulating the factory-gate prices that patentees charge for prescription and non-prescription patented drug products sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, to ensure that they are not excessive. The PMPRB regulates the price of each patented drug product (each strength of an individual, final dosage form of a drug). This is normally the level at which Health Canada assigns a Drug Identification Number (DIN) as part of the Notice of Compliance process. However, the Board's mandate also includes drug products available under the Special Access Programme; drug products available through a Clinical Trial Application; and Investigational New Drug Products.
The Federal Court of Appeal articulated the legal requirement as to when a patent will "pertain" to the medicine. In this regard, the Court established the "merest slender thread" requirement, which is wide in scope. The Board's jurisdiction is not limited to drug products for which the patent is on the active ingredient. Rather, the Board's jurisdiction also covers drug products for which the patents relate, but are not limited, to the processes of manufacture, the delivery system, dosage form, indication/use, and/or any formulation. Patented drug products are not limited to brand name drug products. A number of generic companies fall under the Board's jurisdiction by virtue of being licensees (i.e. authorized to sell the same drug product as the brand company is selling) or due to their own patents (e.g. related to processes of manufacture).
The PMPRB has no authority to regulate the prices of non-patented drug products and does not have jurisdiction over prices subsequently charged by wholesalers or retailers or over pharmacists' professional fees. In addition, matters such as whether drugs are reimbursed by public drug plans, distribution channels and prescribing are outside the purview of the PMPRB.
Under the Act, patentees are required to inform the PMPRB of their intention to sell a new patented drug product. Upon the sale of such a patented drug product, as per the Patented Medicines Regulations, patentees are required to file price and sales information for the first day's sales and, thereafter, twice a year for six month periods (January to June and July to December) for each strength of each dosage form of each patented drug product sold in Canada for price review/regulation purposes, for the duration of the patent(s).
Although patentees are not required to obtain the PMPRB's approval of the price of a patented drug product before it is sold, they are required to comply with the Act to ensure that prices of patented drug products sold in Canada are not excessive. If a patented drug product is sold before the patent issues, the PMPRB will review the price of the product as of date of first sale, as long as this is after the date on which the patent application was laid open for public inspection.
In the event that the Board finds, after a public hearing, that a price is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting Role
The PMPRB reports annually to Parliament, through the
Minister of Health, on its activities, on pharmaceutical trends relating to all patented drug products, and on the R&D spending
by pharmaceutical patentees. In addition to these reporting responsibilities, under section 90 of the Act the Minister of
Health has the authority to direct the PMPRB to inquire
into any matter. Under this provision, the Minister has directed the Board to undertake two initiatives: the National Prescription
Drug Utilization Information System (NPDUIS), and monitoring and reporting on Non-Patented Prescription Drug Prices (NPPDP).
National Prescription Drug Utilization Information System (NPDUIS)
Since 2001, pursuant to an agreement by federal, provincial and territorial Ministers of Health, the
PMPRB has been conducting research under the
NPDUIS. The purpose of the
NPDUIS is to provide critical analyses
of price, utilization and cost trends so that Canada´s health system has more comprehensive and accurate information on
how prescription drugs are being used and on sources of cost drivers.
Non-Patented Prescription Drug Prices (NPPDP)
In 2005, the Minister of Health, on behalf of federal, provincial and territorial Ministers of Health, directed the
PMPRB to monitor and report on non-patented prescription
drug prices. This function is aimed at providing a centralized credible source of information on non-patented prescription
drug prices. Since April 2008, NPPDP studies are conducted
under the umbrella of the NPDUIS.
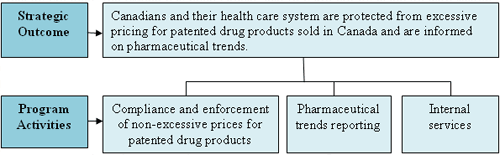
Strategic Outcome and Program Activity Architecture
The PMPRB has one Strategic Outcome (SO) and three program activities (PAs) — representing its regulatory and reporting roles — which are illustrated in the following chart:
Planning Summary
| 2010-2011 | 2011-2012 | 2012-2013 |
|---|---|---|
| $12,181.6 | $11,816.2 | $11,816.2 |
| 2010-2011 | 2011-2012 | 2012-2013 |
|---|---|---|
| 761 | 76 | 76 |
Planning Summary Table ($ thousands)
| Performance Indicator | Target |
|---|---|
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. | Canada's prices on average are at or below the median of international prices. |
| Program Activity | Forecast Spending 2009-2010 |
Planned Spending | Alignment to Government of Canada Outcomes | ||
|---|---|---|---|---|---|
| 2010-2011 | 2012-2012 | 2012-2013 | |||
PA 1: |
$5,572.7 |
$7,648.8 |
$7,654.3 |
$7,654.3 |
Healthy Canadians |
PA 2: |
$992.5 |
$1,624.8 |
$1,626.6 |
$1,626.6 |
Healthy Canadians |
PA 3: |
$3,333.6 |
$2,908.0 |
$2,535.3 |
$2,535.3 |
|
Total Planned Spending |
$9,898.8 |
$12,181.6 |
$11,816.2 |
$11,816.2 |
|
Contribution of Priorities to Strategic Outcome
Operational Priorities
| Operational Priority | ||
|---|---|---|
| Implement and monitor the revised Excessive Price Guidelines (Guidelines), policies and procedures. | Type: New | Links3 to Program Activity 1 |
Why is this a priority? The PMPRB is responsible for ensuring that the prices that patentees charge, the "factory-gate" price, for patented drug products sold in Canada for human and veterinary use, are not excessive. The PMPRB relies on voluntary compliance whenever possible since it is less time consuming and less costly to all parties than hearings. Voluntary compliance by patentees is facilitated by published Guidelines on excessive prices, which are intended to assist patentees in setting prices that are not excessive by providing transparent and predictable information on how the price review will be carried out. The Guidelines are published in the PMPRB's Compendium of Policies, Guidelines and Procedures (Compendium) and are available on the Web site http://www.pmprb-cepmb.gc.ca, under Legislation, Regulations, and Guidelines. In order to ensure the Guidelines remain appropriate and effective in the modern pharmaceutical environment and that they also uphold the principles of fairness, transparency, openness and predictability, the Board initiated a process in 2005 to review its Guidelines, including consulting with key stakeholders as required by the Act. Revised Guidelines were released in June 2009 for implementation starting January 1, 2010. It is essential to monitor and evaluate the application and impact of changes to the Guidelines to ensure anticipated intents are being achieved. Plans for meeting the priority
|
||
| Operational Priority | ||
|---|---|---|
| Redevelop the Compliance Database. | Type: Previously committed to | Links to Program Activities 1 and 3 |
Why is this a priority? The database housing price and sales information filed by patentees is a key tool for the conduct of regulatory activities. The twenty-year-old database requires redevelopment to:
Plans for meeting the priority
|
||
| Operational Priority | ||
|---|---|---|
| Ensure hearing processes are transparent and more efficient. | Type: Previously committed to | Links to Program Activity 1 |
Why is this a priority? Hearings have increased significantly in number and complexity in recent years. The ability to hold public hearings when needed is a core component of the Board's mandate and authority. As a quasi-judicial body, the PMPRB must give patentees a fair and timely hearing, as needed. Plans for meeting the priority
|
||
| Operational Priority | ||
|---|---|---|
| Enhance the profile and uptake of the research and analysis conducted by the PMPRB. | Type: New | Links to Program Activity 2 |
Why is this a priority? Board members and senior management require high quality research and analysis on which to base policy decisions. The PMPRB also needs to ensure that research and analysis conducted through the NPDUIS initiative continue to address the needs and priorities of participating federal, provincial and territorial public drug plans. Other external audiences and stakeholders may also benefit from a greater awareness of the PMPRB's research and analysis related to pharmaceutical trends. Plans for meeting the priority
|
||
Management Priorities
| Management Priority | ||
|---|---|---|
| Strengthen internal capacity of Board Staff and the Board by staffing vacant positions. | Type: New | Links to Program Activities 1, 2 and 3 |
Why is this a priority? Reference level adjustments have been made to address workload impacts arising from changes to the Guidelines, emerging scientific and price review issues and an increasing volume of regulatory filings, price reviews, investigations and hearings. The PMPRB shares with other small organizations the difficulty in attracting and retaining highly specialized subject matter experts. The organization made significant progress in staffing new positions in 2009-2010, but significant work remains. In addition, the PMPRB will experience some turn-over at the senior management level as the Executive Director is expected to retire, and the terms of two Governor-in-Council (GIC) appointees will expire. Plans for meeting the priority
|
||
| Management Priority | ||
|---|---|---|
| Develop and implement IM plans and policies that will enhance capabilities to meet operational and reporting needs for information. | Type: New | Links to Program Activities 1 and 3 |
Why is this a priority? The effective management of information is critical to the PMPRB's performance, from a regulatory perspective with respect to compliance and enforcement, and with respect to the public interest. A growing PMPRB organization, the number of hearings and increased performance management reporting requirements have changed the PMPRB's information management needs. Plans for meeting the priority
|
||
| Management Priority | ||
|---|---|---|
| Prepare for evaluation of Compliance and Enforcement Program in 2011-2012. | Type: New | Links to Program Activity 3 |
Why is this a priority?
Plans for meeting the priority
|
||
Risk Analysis
In recent years we have witnessed important economic, social, demographic and technological changes which have impacted Canadian health care and the pharmaceutical environment.
The PMPRB's capacity to carry out its statutory regulatory mandate is focused on its ability to conduct timely price reviews, investigations and, when needed, hearings. From its creation until 2005, the PMPRB had been largely able to carry out its mandate with limited recourse to public hearings due, at least in part, to the success of the Board's Excessive Price Guidelines (Guidelines) and its Voluntary Compliance Policy.
In 2005-2006, the PMPRB identified a risk under its regulatory mandate stemming largely from an unprecedented number of investigations and anticipated hearings into excessive drug prices. This, coupled with renewed stakeholder attention to drug prices and concerns about high introductory drug prices and other issues, prompted the Board to initiate a comprehensive process to review its Guidelines, including consulting with key stakeholders as required by the Patent Act (Act).
This review was aimed at ensuring the effectiveness, fairness, transparency and predictability of the price review process. The consultations, along with the input of five working groups, were aimed at determining whether, where and how the Guidelines should be updated to be more appropriate and relevant in today's modern pharmaceutical environment. The Board released its new Excessive Price Guidelines on June 9, 2009, with an implementation date of January 1, 2010.
The PMPRB may experience some challenges related to operationalization of the revised Guidelines. The increased complexity of scientific reviews (due to additional therapeutic improvement factors) and price reviews (including new reviews at the level of sub-markets and a new CPI "Delinking" methodology when price increases are due to changes in benefits for customers) will add to workload demands. The revised Guidelines may lead to more investigations and hearings as patentees adjust to the revised Guidelines, although in the long run it is expected that the new Guidelines will enhance voluntary compliance. In addition, workload pressures will likely continue, for at least the short term, with respect to more complex hearings. These areas will need to be closely monitored and assessed with adjustments in capacity and process, and potentially even the Guidelines, undertaken as needed.
Resource requirements have been identified to monitor and evaluate the impact of the revised Guidelines and to analyze and provide advice on emerging scientific and price review issues. Board Staff will undertake these activities while managing the increasing volume of regulatory filings, carrying out price reviews and investigations within reasonable timelines, and participating in hearings in a reasonably expeditious manner. Staff will also be required to participate in a major information technology renewal project. Provisions were made in the 2009-2010 adjustment to the PMPRB's Annual Reference Level Update (ARLU); however, significant attention will need to be paid to the allocation of these resources, especially given the difficulty in projecting actual workload impacts arising from changes to the Guidelines.
The PMPRB shares with other small organizations the difficulty in attracting and retaining highly specialized subject matter experts and the length of time required to engage new staff. The organization made significant progress in staffing new positions in 2009-2010, but significant work remains. Training must also be provided to address skill and knowledge gaps.
In addition, the PMPRB will experience some turn-over at the senior management level due to retirement, and will welcome new Board members as their terms expire.
The opportunity, and the challenge, over the planning period will be to ensure that human resource planning and business planning remain in alignment, and that operational staffing activities and other support services are equipped to support the evolving organizational needs and directions. The PMPRB's integrated business and human resources planning framework ensures the identification, review and documentation of human resources requirements on a quarterly basis, and frequent review of the status of staffing activities. Increased capacity in Human Resources will enhance support to program areas.
For the most part, matters before the Board have focused on the scientific and price issues related to brand name patented drug products. Some more recent cases have also centred on the Board's jurisdiction, for example, with regard to drugs sold from outside the country through Health Canada's Special Access Programme, and to patented generic drug products. While proceedings before the Board are time sensitive, resource intensive, and require dedication and thoughtful deliberation, they also provide patentees with an opportunity to be heard by the Board on issues vital to their operations. Board proceedings have, in some cases, resulted in judicial review applications before the Federal Court and the Federal Court of Appeal, which ultimately provide both the Board and patentees with clarification on key legal issues but may give rise to changes in the scope and conduct of the Board's regulatory mandate.
Expenditure Profile
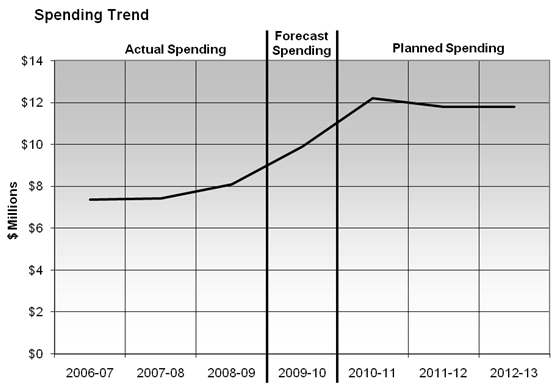
In recognition of increasing workload pressures, the PMPRB was provided program integrity funding in the amounts of $4.9 million for 2006-2007 (in Supplementary Estimates A) and $5.0 million for 2007-2008 from the Treasury Board Risk Management Reserve to augment its $5 million A-base budget.
Those amounts included $4.4 million and $3.2 million respectively for hearings in 2006-2007 and 2007-2008, which were placed in an existing Special Purpose Allotment (SPA) in the amount of $300 thousand. The ability to hold public hearings when needed is a core component of the Board's mandate and authority. Due to the difficulty in forecasting the number and complexity of hearings in any given year, the amounts related to external hearing costs (legal counsel, expert witnesses, etc.) are placed in the SPA so that they are reserved strictly for that purpose. Any unspent amount lapses at year end.
In anticipation of the sunsetting of the temporary program integrity funding in 2008-2009, the PMPRB was granted permanent funding in the amount of $4.7 million, in addition to the core A-base of $5.8 million, to enable the PMPRB to meet its workload pressures and continue ongoing initiatives related to the delivery of its mandate.
Treasury Board approved authority to increase reference levels in Vote 35 (Program expenditures) by $5.6 million for 2009-2010, $6.2 million for 2010-2011, and $5.8 million for 2011-2012 and future years (including EBP and excluding Public Works and Government Services Canada accommodation charges).
As part of these increased resources, authority was given to increase the Special Purpose Allotment of $300 thousand to conduct Public Hearings in Vote 35 (Program Expenditures) by $1.9 million in 2008-2009, $2.2 million in 2009-2010, $2.8 million in 2010-2011, and $2.8 million in 2011-2012 and future years. This was intended to provide assurance that the PMPRB would have the necessary resources to hold hearings even in the most exceptional years.
Although expenditures (i.e. actual) rose each year from 2006-2007 to 2008-2009, there were fewer hearings than anticipated. Some were settled through the submission of Voluntary Compliance Undertakings (VCUs). In addition, the staffing of some positions has presented a challenge. The PMPRB expects to report expenditures of $9.6 million from an expected final reference level of $11.8 million in 2009-2010.
| Vote # or Statutory Item (S) | Truncated Vote or Statutory Wording | 2009-2010 Main Estimates |
2010-2011 Main Estimates |
|---|---|---|---|
| 35 | Program expenditures | $10,369.0 | $11,163.3 |
| (S) | Contributions to employee benefit plans | $989.0 | $1,018.3 |
| Total PMPRB | $11,358.0 | $12,181.6 | |
