Common menu bar links
Breadcrumb Trail
ARCHIVED - Hazardous Materials Information Review Commission Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
President's Message
As President and Chief Executive Officer of the Hazardous Materials Information Review Commission (HMIRC), I am pleased to submit to Parliament, and to Canadians, the Commission's 2010-2011 Report on Plans and Priorities. This report outlines our goals and objectives for the next three years in support of improved worker health and safety and the protection of the chemical industry's trade secrets within the Workplace Hazardous Materials Information System (WHMIS). Through innovation and streamlining, the Commission will be able to exploit its knowledge and expertise in the field of hazard classification and communication in the Canadian workplace to the benefit of all stakeholders.
Following up on our legislative renewal, HMIRC will continue to review and revise the claims exemption process in order to provide an effective and efficient service. This will involve enhancements to client support services, such as tools to boost proactive compliance in the preparation of hazard communication and to assist in the future implementation of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). As well, the three-year claims backlog elimination plan is on target and new procedures and instruments are in place to ensure future workload capacity.
HMIRC will also explore ways to increase the organization, translation and dissemination of its scientific knowledge at the national and international levels and across industry and labour. This will include a better understanding and identification of methods to improve the quality of Material Safety Data Sheets (MSDSs), the development of more preventive and educational approaches to effective hazard communication, and partnerships with key organizations. The Commission will continue to participate in discussions on the implementation of GHS in Canada as well as on changes to the Hazardous Products Act and Controlled Products Regulations.
With the support of the Council of Governors, the Commission has embarked on the development and implementation of an integrated plan which will align strategic and operational objectives across the organization. A logic model and an evaluation framework will also be put in place. These activities will sustain the management and accountability of the HMIRC's resources and our commitment to Treasury Board's Management Accountability Framework. The resultant organizational coherence will facilitate the fulfilment of the Commission's mandate and the achievement of its strategic outcome.
I very much appreciate the ongoing commitment and hard work of Council and the Commission staff with respect to our current activities and future endeavours. I look forward to building on our success in contributing to strengthening WHMIS in the workplace and supporting Canadian industry.
Sharon Watts
President and Chief Executive Officer
Section I: Commission Overview
Summary Information
Raison d'être
The Hazardous Materials Information Review Commission provides a single mechanism under federal, provincial and territorial legislation to protect the trade secrets of companies that supply or use hazardous materials, and ensures that Canadian workers who handle such materials have all the information they need to do so safely.
Mandate
The Hazardous Materials Information Review Act mandates the Commission to:
- Register claims for trade secret exemptions and issue registry numbers;
- Issue decisions on the validity of claims for exemption on the basis of prescribed regulatory criteria;
- Make decisions on the compliance of Material Safety Data Sheets (MSDSs) and labels with Workplace Hazardous Materials Information System (WHMIS) requirements;
- Convene independent boards to hear appeals from claimants or affected parties.
Responsibilities
The Commission enables companies to protect their trade secrets and, at the same time, ensures that MSDSs for products with trade secrets used by workers in Canada disclose complete and accurate information to reduce workplace-related illness and injury. The Commission's activities are key components of WHMIS, which was created in 1987 through a consensus of labour, industry and government. The success of WHMIS depends on cooperation among all these partners. All three groups play an integral part in ensuring that chemical products are used as safely as possible in Canadian workplaces.
WHMIS requires that suppliers provide employers with information on the hazards of materials sold for use in Canadian workplaces. The employers, in turn, provide that information to workers through product labels, worker education programs, and MSDSs. A product's MSDS must fully disclose all hazardous ingredients in the product, its toxicological properties, the safety precautions workers need to take when using the product, treatment required in the case of injury, and other pertinent information.
When a supplier introduces a product and wants to protect the identity of one or more of the hazardous ingredients or the concentration, the company needs to apply to HMIRC for an exemption from the requirement to list all hazardous ingredients on the product's MSDS. When HMIRC receives a complete application, HMIRC registers the claim and the product can be made available in the marketplace without disclosing the confidential business information. The Commission then evaluates the claim and issues a decision on its validity and, to protect workers, verifies the compliance of the MSDS with the Hazardous Products Act and Controlled Products Regulations. Where areas of non-compliance are identified, claimants are given two options to comply under the Hazardous Materials Information Review Act. First, the Commission offers voluntary compliance undertakings through which to make corrections. If the claimant chooses not to accept the undertaking the Commission issues formal orders obligating the claimant to make the changes.
When an employer purchases a product and wants to protect the identity and/or concentration of any hazardous ingredients, or the name and the supplier of the product, the company also needs to apply to HMIRC for an exemption. In this case the Commission evaluates the MSDS and if necessary, the label, against the requirements of either the Canada Labour Code , for federally regulated employers, or the relevant provincial or territorial occupational health and safety regulations.
In cases where there are disputes that can not be resolved, HMIRC convenes independent boards to hear appeals from claimants or affected parties challenging decisions and orders or from affected parties challenging undertakings signed by claimants and accepted by HMIRC.
In addition, HMIRC responds to requests from federal, provincial or territorial government health and safety officials for information about claims for exemption to help these officials administer and enforce their WHMIS obligations.
Cooperative Partnerships
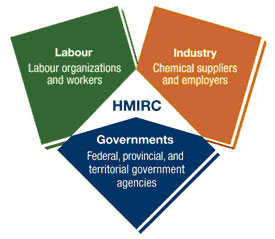
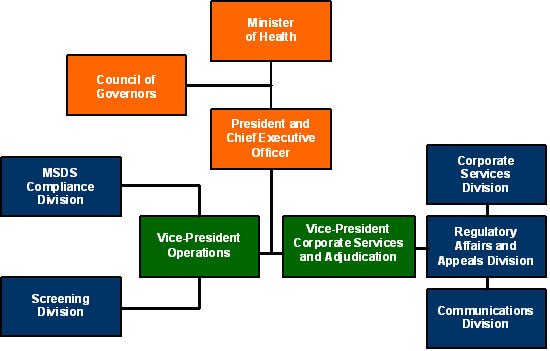
Governance Structure
The Commission's governance structure is a model of collaboration between government, industry and labour. The Council of Governors provides strategic advice and guidance to the Commission and makes recommendations to the Minister of Health. It consists of up to 18 members representing key stakeholders across all jurisdictions:
- 2 representing workers,
- 1 representing suppliers,
- 1 representing employers,
- 1 representing the federal government, and
- Up to 13 representing the provincial and territorial governments.
The Commission's President and Chief Executive Officer is appointed by the Governor in Council and has the authority to supervise and direct the organization's day-to-day activities.
The Vice-President of Operations directs the work of the MSDS Compliance and Screening divisions.
The Vice-President of Corporate Services and Adjudication directs the work of the Corporate Services, Regulatory Affairs and Appeals, and Communications divisions.
Values and Operating Principles
The Commission recognizes that continuous improvement is critical in order to remain relevant and to provide effective and efficient performance and service quality. HMIRC has identified the values and operating principles that foster continuous improvement in its operations. These are: FAIRNESS, TIMELINESS, ACCESSIBILITY, TRANSPARENCY, ACCOUNTABILITY, QUALITY, CONSISTENCY, COMPETENCY, RESPECT, SECURITY and CONFIDENTIALITY.
The Commission's values and operating principles are aligned with and uphold the values of the Public Service as expressed in the Values and Ethics Code:
http://www.tbs-sct.gc.ca/pubs_pol/hrpubs/tb_851/vec-cve1-eng.asp#_Toc46202800
Strategic Outcome and Program Activity Architecture (PAA)
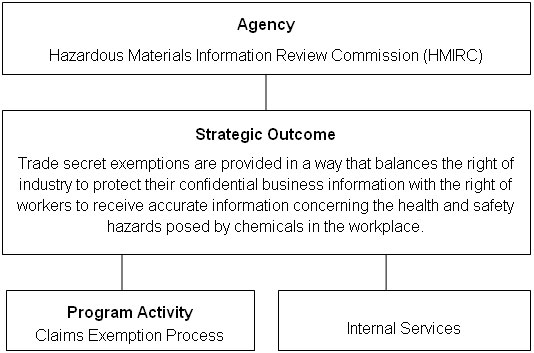
Planning Summary
| 2010-11 | 2011-12 | 2012-13 |
|---|---|---|
| 5,704 | 4,545 | 4,545 |
| 2010-11 | 2011-12 | 2012-13 |
|---|---|---|
| 54 | 42 | 42 |
| Performance Indicator | Targets |
|---|---|
| Number of appeals as a percentage of total claims processed | Zero |
| Program Activity | Forecast Spending 2009-10 |
Planned Spending | Alignment to Government of Canada Outcomes | ||
|---|---|---|---|---|---|
| 2010-11 | 2012-12 | 2012-13 | |||
| Claims Exemption Process | 5,786 | 4,232 | 3,090 | 3,090 | Healthy Canadians |
| Internal Services | - | 1,472 | 1,455 | 1,455 | Healthy Canadians |
| Total Planned Spending | 5,704 | 4,545 | 4,545 | Healthy Canadians | |
1 As indicated in discussions with TBS, the Commission will undertake a review of its Strategic Outcome (SO) and Program Activity (PA), including expected results, performance indicators, and targets, to ensure that the SO and PA support the strategic direction established by the 2009-2012 Strategic Plan.
Contribution of Priorities to Strategic Outcome
| Operational Priorities | Type | Links to Strategic Outcome(s) | Description |
|---|---|---|---|
| Priority 1: Enhanced approach to support proactive compliance |
Ongoing | Strategic Outcome 1 | The Commission will continue to enhance compliance support to claimants, particularly in
the area of MSDSs and product labels. The Commission will continue to review its claims process and to identify innovative and efficient ways to improve it. Tools will be developed or revised to provide better information and guidance to claimants regarding hazard classification and communication. |
| Priority 2: Improved knowledge translation to support hazard classification and communication |
Ongoing | Strategic Outcome 1 | HMIRC will contribute knowledge and expertise acquired from its organizational experiences
to stakeholder discussions on improving hazard classification and communications systems in Canada. The Commission will acquire knowledge and expertise on the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) in order to be in an optimal position to support claimants to attain higher MSDS compliance as a result of the changes brought about by Canada's response to the GHS. This will also support proactive compliance. The Commission will explore educational, preventive and interactive approaches in its work to promote hazard communication. |
| Priority 3: Increased partnerships to better address hazard communication challenges |
Previously committed to | Strategic Outcome 1 | HMIRC will maintain its tripartite collaboration with federal, provincial and territorial
government bodies, industry, and labour. It will identify new opportunities for partnerships with key stakeholders. |
| Management Priorities | Type | Links to Strategic Outcome(s) | Description |
|---|---|---|---|
| Priority 4: Organizational cohesion and coherence |
New | Strategic Outcome 1 | The Commission will continue the design and implementation of an integrated planning process which will enable it to incorporate and align organization-wide and unit-specific planning efforts in order to maximize its desired impact. The integrated plan consists of strategic directions developed with and approved by the Council of Governors and presented to HMIRC staff, an operational plan which supports the strategic directions, an evaluation framework to evaluate the results of activities and other enablers, such as an HR Strategy to ensure the proper resources are in place to attain the desired strategic outcomes. |
Risk Analysis
Operational Context
Labour, industry and government agree on the importance of preventing illnesses and injuries from hazardous materials in Canadian workplaces. In order to help achieve this goal WHMIS was created through the adoption of laws and regulations and the development of procedures in the late 1980s. WHMIS requires suppliers, including manufacturers, importers and distributors, and employers, to provide health and safety information about the chemicals produced or used in Canadian workplaces.
As part of the WHMIS initiative, the Hazardous Material Information Review Act and its regulations also came into force. This legislation established the Hazardous Materials Information Review Commission (HMIRC), an independent agency with a quasi-judicial role. The Commission provides the mechanism in Canada to protect the confidential business information of chemical suppliers and employers and to ensure accurate and complete health and safety information is available to workers.
Risks and Opportunities
The Commission's commitment to occupational health and safety is the impetus for initiatives such as the Backlog Elimination Plan and the handling of priority claims identified by their potential hazards. The backlog is expected to be eliminated by the end of 2010-2011.
While acknowledging an improvement in compliance, ongoing and innovative effort will be made to reduce the percentage of MSDS violations; however, consultations with stakeholders suggest that the levels of violations (i.e., non-compliance) in MSDSs that do not contain trade secrets and therefore are not reviewed by the Commission could be quite high, although evidence is lacking to support this assessment.
The approaching implementation of GHS in Canada will impact hazard communication across WHMIS, including HMIRC. In order to contribute to the transmission of accurate and consistent information, the organization will need to continue to augment its knowledge base, participate in implementation discussions and keep informed of national and international developments. The Commission has a role to play in keeping its stakeholders aware of GHS implications.
The Commission is currently involved in developing an integrated plan. It has sought the input and approval of the Council of Governors and consultation with HMIRC staff. It will be up to management to carefully apply the Commission's modest resources to achieve a coherent alignment of corporate and operational objectives. This will help address persistent challenges such as the recruitment and retention of scientific staff. Ultimately, this exercise will strengthen service and accountability.
Expenditure Profile
In 2007-08, HMIRC developed a plan to eliminate its backlog of claims for exemption over a three year period (2008-2011) and to prevent it from recurring. However, due to its very small A-Base, which in fiscal year 2007-2008 totaled $3.5 M, the Commission sought supplementary funding for each of the three years, as well as ongoing funding, for 2011-2012 and beyond, to implement the plan.
In January 2008, Health Canada (HC) and HMIRC, in consultation with the Treasury Board Secretariat, and with concurrence of the Minister of Health, agreed that Health Canada would transfer $1.7 M to HMIRC to begin the work required to address the backlog through the 2008-2009 Supplementary Estimates A. The Annual Reference Level Update was identified as the appropriate mechanism for transferring supplementary funding of $2 M each year for 2009-2010 and 2010-2011, as well as $850K for 2011-2012 and ongoing.
The figure below illustrates HMIRC's actual spending from 2006-2007 to 2008-2009, forecast spending in 2009-2010, planned spending in 2010-2011 to 2012-2013, and the sunsetting of funding to address the claims backlog.
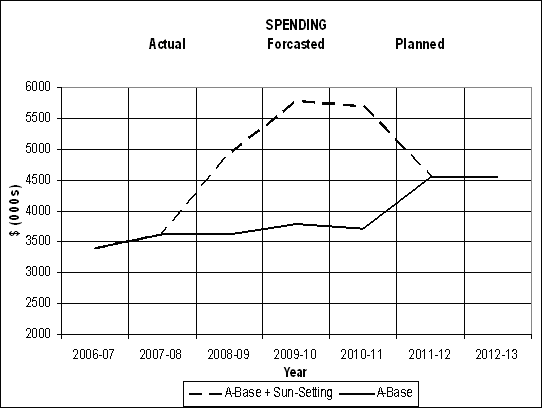
| Vote # or Item (S Statutory) | Truncated Vote or Statutory Wording | 2009-10 | 2010-11 |
|---|---|---|---|
| 30 | Program expenditures | 4,855 | 4,980 |
| (S) | Contributions to employee benefit plans | 700 | 724 |
| Total | 5,555 | 5,704 | |
