Common menu bar links
Breadcrumb Trail
ARCHIVED - Canadian Institutes of Health Research
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Section II - Analysis of Program Activities by Strategic Outcome
Introduction
CIHR's revised Program Activity Architecture (PAA) effective 2008-2009 is shown in Figure 3. The PAA consists of three strategic outcomes and the key program activities (and sub-activities) that report them. The information on CIHR's planned programs and activities presented in the following pages is organized according to this structure.
Figure 3:
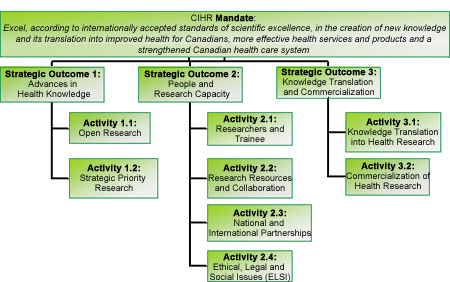
1. Strategic Outcome: Advances in Health Knowledge
CIHR's Strategic Outcome 1.0 ensures that:
the Canadian health research advances health knowledge and is responsive to current opportunities and priorities
| Expected Result | Indicators |
|---|---|
| New health research knowledge available in areas of opportunity and priority. |
|
CIHR supports the development of new knowledge through health research across all disciplines that are relevant to health. Throughout 2008-2009 and beyond, CIHR will continue to support health research in order to create health knowledge responding to opportunities and priorities.
1.1 Program Activity: Open Research
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $462.6 | $464.3 | $464.3 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 228 | 232 | 231 |
Program Summary
Open research enables the conduct of health research in emerging areas of science across all disciplines that are relevant to health. This is achieved through managing and launching competitions, based on internationally accepted standards of scientific excellence and a peer review process, to fund grants open to all areas of health research.
| Expected Results | Indicators |
|---|---|
| Excellent health research conducted responding to best researcher ideas, through effective funding programs. |
|
| Link to Priority | |
| Strategic Priority #1: Research - Advance health knowledge, through excellent and ethical research, across disciplines, sectors, and geography. | |
Description of Key Programs and Services
Research driven by the creativity of individuals lies at the heart of Canada's health research enterprise. CIHR encourages and promotes excellence in research, as judged by peers, recognizing that innovative research drives progress and ensures a continuous flow of fresh insights. Over the next three years, CIHR will reinforce its commitment to
research excellence by directing the majority of its base budget to grants and awards in support of investigator-initiated research programs that address significant questions in biomedical and clinical research as well as in the areas of health systems and population and public health research.
Funding Excellence in Health Research: CIHR's Open Operating Grants Program
The Open Operating Grant Program provides operating funds to support research proposals in all areas of health research. This program, which supports excellence in research as evaluated through the peer review process, is the foundation of CIHR's programming. Competitions are held each March and September with an open call for investigator-initiated research
proposals, with no restrictions on areas of research, team size/composition, or maximum level of requested funds.
| In 2008-2009, INMHA will begin funding its Planning & Development for Mental Health in the Workplace initiative. This partnership will involve co-funding with the Health Canada's Employee Assistance Services Bureau, Alberta Centre for Child, Family and Community Research, BC Mental Health and Addictions Research Network, IHDCYH, IHSPR and will offer 1 year funding for grants. |
This program represents CIHR's single largest investment, with a 2008-2009 program budget accounting for more than half of CIHR's grants and awards base budget. This program encourages Canadian health researchers to pursue their very best ideas, define and pursue the mode of research best suited to advance those ideas, and to pursue the opportunities most likely to maximize the impact of their work. This program recognizes the reality that the pursuit of excellence in research, as judged by peers, is a powerful source of inspiration. This program is an important part of CIHR's mandate and has been applauded by governments and research funding agencies worldwide.
In 2008-2009 CIHR will continue to reinforce its long-term commitment to this program through maintaining a budget of at least $378 million, making improvements to the peer review process that supports it, and improving multi-year planning to ensure that new ideas and new researchers are given the opportunity to flourish with each annual competition cycle.
Randomized Controlled Trials Program
The Randomized Controlled Trial (RCT) program, with a 2008-2009 budget of $30.7 million, supports experiments involving groups of randomly assigned human subjects to receive or not receive one or more interventions that are being compared. The results are analyzed by comparing outcomes within and among the groups. This program
provides high quality evidence on the efficacy and effectiveness of interventions in health and health services.
The main function of the RCT unit is to manage the open research competition cycle of submissions to its annual competitions. The specific functions carried out by the unit are: overseeing application processing, competition management and post-award administration; staffing the peer review panels; coordination and management of the Under Continuing Review (UCR) process; and coordination and management of Randomized Controlled Trial Registration Initiative. Closely linked to the main business of this unit is managing oversight of high risk trials, and managing a mentoring program. Oversight is the process whereby CIHR sits as an ex officio member of a trial's Steering Committee. In this position CIHR can serve as a resource to the investigators on matters of agency policy and procedure. As well, CIHR will have early awareness of successes or problems in the execution of the funded work.
For 2008-2009, a recently established RCT Working Group, chaired by Dr. Michael Kramer, will be submitting a report designed to improve the functions of the RCT program. For example, some recommendations will focus on how to enhance the proportion of applications that are funded and mechanisms to enhance promotion of the results of completed trials.
| In 2008-2009, IAPH will finalize its Suicide Prevention initiative. This partnership with Health Canada's First Nation and Inuit Health Branch and INMHA will fund grants in the area of suicide prevention targeting aboriginal people with an emphasis on a multidisciplinary approach. |
Team Grant Program
The Team Grant program supports large teams of talented and experienced researchers conducting high-quality research in all areas of health research and providing superior research training and mentorship. The program emphasis is on the production of new knowledge through collaborative problem-based research involving teams of at least three principal investigators. These results will
be realized more rapidly and more efficiently through the CIHR Team Grant program than if the researchers were to be funded as a series of separate operating grants.
In 2008-2009, through the third annual call for proposals CIHR will fund approximately 10 new teams focused on resolving health issues of high importance to Canadians. Biennial competitions are planned hereafter. In addition to the open or "untargeted" team grant program, targeted competitions for teams and emerging teams continue to be launched to encourage researchers to join together into unique teams to focus on resolving some of the most difficult and complex health problems, including pandemic preparedness, clinical imaging, and injury prevention, treatment and control.
1.2 Program Activity: Strategic Priority Research
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $122.6 | $104.6 | $107.4 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 60 | 52 | 53 |
Program Summary
Funding strategic priority research enables CIHR to address strategic health opportunities, threats and challenges to Canadians, identified in consultation with health research partners and aligned with government priorities. This is achieved through managing and launching competitions to fund grants in targeted priority health research areas.
| Expected Results | Indicators |
|---|---|
| Excellent health research conducted responding to research priorities, through effective funding programs. |
|
| Link to Priority | |
| Strategic Priority #1: Research - Advance health knowledge, through excellent and ethical research, across disciplines, sectors, and geography. | |
Description of Key Programs and Services
CIHR commits approximately 30% of its grants and awards base budget to its various strategic initiatives. These strategic initiatives respond to health challenges that are relevant to government priorities and are of high priority to Canadians.
Strategic Priority Operating Grant Program
The Strategic Priority Operating Grant Program is led by CIHR's 13 Institutes and funds operating grants to support research in priority areas to address strategic health opportunities, threats and challenges to Canadians. These strategic priority research areas are identified by the Institutes in consultation with
stakeholders from government, health care organizations, patient groups, and industry. Proposals are solicited from researchers by issuing a funding opportunity outlining the specific theme/area where research is needed, at which point each application is reviewed by a peer review committee using specific selection criteria. For 2008-2009, CIHR's Institutes and
partners will be funding strategic operating grants in at least 25 different priority areas.
Large Strategic Initiatives Program
The Large Strategic Initiatives program supports operating funds for large strategic initiatives, which involve a joint, cross-cutting effort involving two to thirteen of the CIHR Institutes. These strategic research initiatives are identified by the Institutes in consultation with stakeholders from government, health
care, patient groups, and industry.
CIHR's Strategic Plan, Blueprint, calls upon the organization to develop national research platforms and initiatives. CIHR's Governing Council has identified a number of partnered, long-term strategic initiatives to pursue in order to address Canada's health research priorities, such as the following three examples.
Clinical Research Initiative
The priorities of the Clinical Research Initiative (CRI) for 2008-2009 include:
- Developing and sustaining the entire career path of clinical researchers through the provision of career and salary awards;
- Building on and improving clinical research networks to better provide linkages amongst researchers to share expertise and infrastructure;
- Continuing to develop the Clinical Research Operational Training program, to provide a minimum standard of training in Good Clinical Practice and Ethics, for example, to improve the quality of clinical research in Canada and;
- Raising the collective awareness of the importance of clinical research and the impacts this research can have on improving the health care system and the overall health of Canadians.
| In 2008-2009, ICRH will begin funding its Network for Cardiothoracic Surgical Investigations in Cardiovascular Medicine initiative. This partnership with the National Heart, Lung and Blood Institute will offer 5-year grants to conduct clinical research in the area of cardiovascular surgery. |
Also in 2008-2009, CIHR will begin funding Team Grants through the Regional/National Clinical Research Initiatives in partnership with the Canada Foundation for Innovation, with a total value of $150 million over 5 years. The objective is to strengthen clinical research in Canada in order to accelerate the translation of clinical research discoveries into improved and cost-effective approaches to maintaining health and treating illness, and to provide evidence for sound health policies and an efficient health care system.
Regenerative Medicine and Nanomedicine Initiative (RMNI)
Since 2004, the RMNI has provided funding for new and emerging areas of integrative research in nanotechnology and regenerative medicine that span the mandate of CIHR (including research into nanomedicine, stem cells, tissue engineering, rehabilitation sciences, and related social, ethical, environmental, economic, and legal issues). The
scientific knowledge gained from this initiative includes the regeneration and repair of injured tissues and organs, the development of specialized tools and interventions needed to treat diseases and restore function, and the maintenance of health and the prevention of disease.
This integrative model is unique in the world, and reflects CIHR's mandate to bridge all themes of health research. The hope is that by fostering new multidisciplinary research approaches to health problems - in collaboration with other funding agencies, government departments, NGOs and industry - CIHR will help modernize the research enterprise in Canada for the benefit of all Canadians.
The priorities of the RMNI for 2008-2009 include:
- Supporting innovative multidisciplinary research programs through an annual Request for Applications (RFA) competition. CIHR plans to obtain new funding through various sources for an Emerging Team Grant in 2008-2009;
- Promoting an integrated multidisciplinary research community through targeted workshops and conferences. CIHR plans to continue to support the annual Nanomedicine/Nanoforum meeting in 2008-2009, as well as pursue other opportunities;
- Developing evaluation and analysis measures for all RMNI funding programs, to measure their effectiveness and to provide guidance in developing new funding opportunities. Both qualitative and quantitative measures will be examined; and
- Expanding the range of RMNI research funding support by strategically focusing on new research themes with significant partnering opportunities, which includes continuing to seek out new potential funding partners.
Canadian Lifelong Health Initiative
The Canadian Lifelong Health Initiative (CHLI) is a groundbreaking set of large cohort studies targeting birth, chronic disease and aging, that will track the health of thousands of Canadians over many years and generate new knowledge of how environmental, social, life-style, genetic and behavioural factors affect health across the lifespan.
The CHLI comprises the Canadian Longitudinal Study on Aging (CLSA). Much of the activity of the CLSA in 2007-2008 focused on its readiness for the CLSA Field Trial in Spring 2008 in view of the launch of the CLSA Comprehensive Study in October 2008.
For 2008-2009, the priorities will be the launch of the CLSA Comprehensive Study in October 2008, governance, training and marketing. More specifically activities will include:
- Finalizing Standard Operating Procedures (SOPs) and Training Manuals for the cohort participants, nurses, data managers, etc. in time for the launch of the CLSA Comprehensive Study in October 2008;
- Continuing to work closely with the CIHR Ethics Office (and the CIHR-led CLSA ELSI committee) on any ethical, legal and social (ELSI) issues related to documentation and implementation;
- Establishing a CLSA Advisory Committee to advise on the implementation and funding strategies of the future phases of the CLSA and to provide guidance, leadership, advice, and recommendations concerning CLSA activities. They may also advise on scientific and methodological aspects of the CLSA;
- Building awareness and partnerships to exploit CLSA expertise and protocols and to market CLSA to different levels of government, pharmaceutical companies, and research institutes/initiatives. This will require an upgrading of communication efforts and venues. To this end the CLSA principal investigators will following up on discussions with Canadian Partnership Against Cancer (CPAC), CARTOGENE, University of British Columbia Brain Research Centre, Pfizer and Veterans Affairs. Additionally there will be a need to explore marketing venues beyond existing efforts (CLSA newsletter, coordinating CIHR and McMaster Websites, and the Canadian Journal on Aging Supplement reporting on CLSA validations and feasibility studies of phase I of the CLSA); and
- The CLSA Fellowships program will be funding its first fellows. Such awards will build and strengthen expertise in the planning, conduct and analysis of longitudinal studies which assess a broad range of health measures and factors influencing health.
| From 2008 to 2013, through the Cognitive Impairment in Aging Partnership, IA will be supporting the Research to Action Program In Dementia (RAPID): a Network for Translation of Research in Alzheimer Disease and Dementia. RAPID will build capacity not only among emerging scholars to better translate knowledge to the stakeholder community, but also within the stakeholder community to better use research knowledge. Contributing partners are the Alzheimer Society of Canada, the Ontario Ministry of Health and Long-term Care, AstraZeneca Canada Inc., Pfizer Canada Inc., CIHR Knowledge Translation Branch, IGH, INMHA, and CIHR Ethics Office. |
HIV/AIDS Research Initiative
The HIV/AIDS Research Initiative is supported by a targeted investment aimed at supporting HIV/AIDS research across all pillars of health research. The HIV/AIDS Research Initiative is lead by the CIHR Institute of Infection and Immunity (III) and
includes CIHR's involvement in two interdepartmental horizontal initiatives: the Federal Initiative to Address HIV/AIDS in Canada and the Canadian HIV Vaccine Initiative. Four CIHR Institutes, in addition to III, directly contribute to the design and delivery of the
HIV/AIDS Research Initiative through participation in the CIHR HIV/AIDS Research Advisory Committee. These Institutes include IAPH, INMHA, IPPH and IHSPR.
Federal Initiative to Address HIV/AIDS in Canada (Federal Initiative)
This Federal Initiative is a key component of the Government of Canada's response to HIV/AIDS in Canada. The Federal Initiative launched in 2004-2005, aims to strengthen domestic action on HIV/AIDS, build a co-ordinated Government of Canada approach, and support global health responses to HIV/AIDS. Four federal departments and
agencies are responsible for delivering the Federal Initiative, including the Public Health Agency of Canada (PHAC) which acts as the lead department, CIHR, Health Canada and Correctional Service Canada. The Federal Initiative focuses on prevention and access to diagnosis, care, treatment and support for those populations most affected by the
HIV/AIDS epidemic in Canada - people living with HIV/AIDS, gay men, Aboriginal people, people who use injection drugs, inmates, youth, women, and people from countries where HIV is endemic.
The long-term outcomes for the Federal Initiative towards which all involved department/agencies are contributing include:
- Improved health status of persons living with or vulnerable to HIV/AIDS;
- Reduction of social and economic costs of HIV/AIDS to Canadians; and
- Global effort to reduce the spread of HIV/AIDS and mitigate its impact.
Within the Federal Initiative, CIHR is responsible for setting priorities for and administering funding for extramural research. In 2008-2009, key activities for CIHR include finalization of a strategic plan to guide the future directions and investments of the Federal Initiative, funding strategic HIV/AIDS research grants and capacity-building grants and awards, as well as designing and launching new strategic research programs in HIV/AIDS.
Canadian HIV Vaccine Initiative (CHVI)
The CHVI, announced in February 2006, is a collaborative undertaking between the Government of Canada and the Bill & Melinda Gates Foundation to contribute to the global effort to develop a safe, effective, affordable and globally accessible HIV vaccine. Participating federal departments and agencies include PHAC which acts
as the lead department, CIHR, Canadian International Development Agency, Industry Canada and Health Canada.
In 2008-2009, the involved departments and agencies will contribute towards the short-term outcomes for CHVI which include increased and improved collaboration and networking, an enhanced knowledge base, and increased readiness and capacity in Canada.
Within CHVI, CIHR provides scientific leadership and strategic guidance through its linkages to the Canadian research community, as well as brings critical expertise in peer review mechanisms and related professional support services to identify and fund eligible HIV vaccine projects.
Key activities for CIHR in 2008-2009 under CHVI include the establishment of strategic research funding programs to advance HIV vaccines research.
Pandemic Preparedness Strategic Research Initiative (PPSRI)
To ensure that Canada has a coordinated and focused research effort to help prevent or mitigate an influenza pandemic, the Institute of Infection and Immunity (III) established the PPSRI. The PPSRI is supported by the Canadian federal government which announced in May 2006 that it will provide $21.5 million over five years to
support pandemic influenza research.
Guided by its Task Group, the PPSRI's mandate is to identify strategic research priorities and support pandemic preparedness research. The Task Group refined, developed and further prioritized the research areas first identified during the September 2005 Influenza Research Priorities Workshop. The four strategic areas are:
- Vaccines and immunization programs: optimize existing vaccination programs and develop new vaccines and technologies;
- The virus: better understand the biology of the influenza virus and develop rapid diagnostics;
- Prevention and treatment: study modes of transmission, strategies for prevention and optimal use of anti-viral drugs; and
- Ethics, legal and social contract: conduct research in risk communication, resource allocation and the regulatory approval process.
| In 2008-2009, III will finalize its Influenza Research Network initiative which is part of the larger Pandemic Preparedness Strategic Research Initiative. This collaboration with the Public Health Agency of Canada (PHAC) will fund a 3-year Influenza Research Network that will mobilize nation-wide experience and talent in vaccine evaluation. This investment will contribute to the preparedness of the Canadian pandemic and influenza research community and ensure surge capacity and mechanisms for rapid data collection, analysis, and evaluation before and during a pandemic. |
Because it is critical that research addressing these strategic priorities is funded in a timely manner, III has taken a lead role in collaborating with other CIHR Institutes and organizations to initiate funding opportunities for research in these strategic areas.
In 2008-2009, the PPSRI anticipates making commitments to more than $17.5 million in pandemic related research. Key activities for 2008-2009 are:
- To peer review and fund research projects;
- To develop and initiate strategic funding opportunities;
- To hold the First Annual Meeting of Researchers and End-users, which will include participation from PPSRI funded researchers, stakeholders and decision makers to review progress on funded projects, research outcomes and consult on future research needs; and
- To evaluate the implementation phase of PPSRI.
It is anticipated that pandemic research will: help to prevent or mitigate a pandemic; develop better ways to control the spread of influenza; and, provide better treatment to affected individuals. PPSRI will also help develop a strong network of researchers, ensuring Canada has the necessary expertise to respond effectively in the event of a pandemic. This expertise can also be used to assist other countries in crisis.
Expensive Drugs for Rare Diseases Research Initiative
The Expensive Drugs for Rare Diseases (EDRD) initiative is a targeted investment to increase our understanding of the evidence required to inform public reimbursement decisions for expensive drugs for rare diseases, and to gather additional real-world therapeutic effectiveness information.
This initiative is a joint investment by the Government of Canada, participating provinces, and the private sector in a three-year Canadian Fabry Disease Initiative (CFDI) Study, with the objective of gathering additional real-world therapeutic effectiveness information relating to new enzyme replacement therapies for those afflicted with the rare genetic Fabry Disease.
CIHR is responsible for administering the federal funding to the CFDI Study, and is also collaborating with the Fonds de la recherche en santé du Québec on providing scientific leadership for the EDRD Initiative. In 2008-2009, CIHR will be contributing $12.8 million for the CFDI Study and for support of an Independent Scientific Oversight Committee. The Committee will monitor, evaluate and communicate publicly the results of the CFDI research.
National Anti-Drug Strategy Treatment Research Initiative
The National Anti-Drug Strategy Treatment Research Initiative is a targeted investment to support grants for strategic research that will help develop and evaluate drug treatment models and approaches, as part of the Strategy's Treatment Action Plan.
For example, the Institute of Neurosciences, Mental Health and Addiction (INMHA) held a consultation workshop in 2007-2008 where it was decided to launch a Catalyst Grant Program2 in early 2008 devoted only to research priorities related to treatment of illicit drug addiction:
- Understanding factors that encourage illicit drug users to accept and engage in treatment;
- Treatment modalities related to problematic cannabis use;
- Substitution therapies and promising interventions that target specific forms of illicit drugs use; and
- Analysis of the association between the need and the availability of services for drug treatment and the match to best practices.
The deadline for application to this Program is in 2008, and funding is expected to start March 2009. It is expected that up to nine proposals could be funded within the available budget.
A Team Grant Program will also be launched in early 2008. The research priorities will include not only the treatment of illicit drug addiction but also research on alcohol and pharmaceuticals (over-the-counter and prescription) as well as issues related to driving under the influence. Funding for this Program is expected to start in 2009-2010.
2. The Catalyst Grant Program provides seed money, on a short-term basis, to support health research activities which represent a first step towards the pursuit of more comprehensive funding opportunities.
In delivering results related to Strategic Outcome #1, Advances in Health Research, CIHR faces the following risks and challenges:
- The growing volume of applications and the diversity of CIHR programs is putting pressure on the peer review process;
- Concurrently, the high volume of applications and limited CIHR funding is resulting in a situation where a large number of high quality proposals cannot be funded; and
- In addition, the unprecedented expansion of health research capacity across Canada, in particular the investments in state-of-the art infrastructure, is intensifying the demand for CIHR support and the need to better co-ordinate its efforts with other health research funders.
The risk of not adequately addressing these challenges is that CIHR may fall short of providing the strong and diverse research base that is needed for maintaining and improving health and health care in Canada.
CIHR will address these challenges and mitigate these risks with the following strategies:
- Continuing to conduct rigorous, competitive granting process in which only the very best applications are selected for funding, as evaluated by research experts from Canada and around the world;
- Reducing the burden on peer review committees by streamlining our business processes, providing clearer objectives and through the application of technology;
- Informing the research community of funding opportunities through Institute Advisory Boards, delegates in each University, a regular e-bulletin for researchers, and launching them on the CIHR website;
- Rationalizing our programming and consolidating wherever possible funding opportunities; and
- Forging productive relationships with all stakeholders and in particular other funders of the health research enterprise. For example, CIHR will work closely with the Canadian Foundation for Innovation and the other two granting councils to determine thee optimum mix of funding towards infrastructure, operations and research.
2. Strategic Outcome: People and Research Capacity
CIHR's Strategic Outcome 2.0 ensures:
a strong and talented health research community with the capacity to undertake health research
| Expected Result | Indicators |
|---|---|
| A strong and talented health research community with the capacity to undertake health research. |
|
CIHR is committed to strengthening Canada's health research communities by continuing to broaden, deepen and sustain health research excellence. CIHR provides various training and salary programs to support and ensure a strong Canadian health research community that is able to undertake outstanding research in innovative environments.
2.1 Program Activity: Researchers and Trainees
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $196.1 | $200.6 | $201.0 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 97 | 100 | 99 |
Program Summary
This program enables building the capacity of the Canadian health research community by providing the next generation health researchers with training and development support, and providing highly-qualified health researchers with sustained support for scientific careers in health research. This is achieved through managing competitions and programs to fund salary and training awards
for health researchers and trainees.
| Expected Results | Indicators |
|---|---|
| A supply of highly qualified health researchers and trainees is available to conduct excellent research in areas of strength or need as a result of effective funding programs. |
|
| Link to Priority | |
| Strategic Priority #2: Researchers - Develop and sustain Canada's health researchers in vibrant, innovative and stable research environments. | |
Description of Key Programs and Services
Salary Support and Training Programs
Salary Support Program
These programs provide researchers who have reached the stage of independence the opportunity to develop their careers and devote more independent time and effort to initiating and conducting health research by contributing to their salary.
One of CIHR's core objectives is to provide leadership in building capacity within Canada's health research community. This is done through the training and development of researchers, and by fostering the development and ongoing support of scientific careers in health research through the Canada Research Chairs program.
In 2008-2009, CIHR intends to support at least 60 new awards at the New Investigator level, helping to boost the careers of these young researchers.
Training Programs
These programs provide support and special recognition to students who are pursuing a PhD degree or for highly qualified candidates at the post-PhD or post-health professional degree stages in Canada or abroad.
| In 2008-2009, IGH will be funding awards under the Ontario Women's Health Council (OWHC) / IGH Joint Program. This joint program was established to achieve the goal of building women's health research capacity in Ontario. The awards funded under this program create a legacy for the OWHC by seeding women's health research in Ontario, supporting excellent women's health researchers and trainees who will become the next generation of senior-level and independent academics. |
Training the next generation of researchers is crucial to the future of health research in Canada. Demographic trends indicate an increasing need for young researchers. In turn, the health care system depends on research for continual improvements. Trainees not only replenish the ranks of independent investigators, but also help to fill the needs of industry for highly qualified personnel and provide health professionals, financial managers, and policy decision-makers with a research background.
CIHR's regular training awards support more than 2,500 individuals, including undergraduates, Master's and doctoral students, and postdoctoral fellows. CIHR's single largest source of support for research training continues to be its regular research grant programs. With the growth in number and size of these in recent years, there has been a corresponding increase in the number of trainees supported from grants held by researchers, with the current total being more than 5,100. CIHR will continue to explore ways of enhancing the effectiveness and efficiency of these programs.
Canada Research Chairs Grants and the Canada Graduate Scholarships Programs
In collaboration with the other two federal granting agencies (NSERC and SSHRC), CIHR will continue to invest in research capacity building through the Canada Research Chairs program and the
Canada Graduate Scholarships program in 2008-2009 and beyond.
Canada Research Chairs Program
The Canada Research Chairs Program aims to attract and retain some of the world's most accomplished and promising researchers. In 2008-2009 and future years CIHR will continue to provide support for the Chair holders that fall within CIHR's health sciences mandate.
Canada Graduate Scholarships (CGS) Program
The Canada Graduate Scholarships (CGS) Program provides financial support to develop future health researchers at both the Master's and doctoral levels in all health related fields in Canada, providing them with an opportunity to gain research experience. The new funding for this program announced in the 2007 Federal Budget will, over three years, double
the number of CGS that CIHR can support and allow CIHR to fund approximately 400 additional CGS by 2009-2010.
Also, CIHR will be the lead for the tri-agency evaluation of the Canada Graduate Scholarships program, which supports both Master's and doctoral students. This evaluation will be completed in 2008-2009.
| In 2008-2009, IG will be supporting up to another three awards under the Scriver Family MD/PhD Studentships Program in partnership with the Canadian Gene Cure Foundation, along with a generous contribution from Dr. Charles Scriver. The specific objective of this award is to further strengthen the Canadian clinical research endeavour by increasing the number of clinician-investigators working within the IGs mandate. Since 2003, IG has supported 16 awards in partnership with the Canadian Genetic Diseases Network and the Canadian Gene Cure Foundation. |
Strategic Salary Support and Training Programs
In addition to the open salary and training award programs described above, CIHR's Institutes and partners support additional trainees and independent researchers in areas where they have determined there is a need to attract and support trainees and world-class independent researchers. These strategic priority research areas are identified by the Institutes in
consultation with stakeholders from government, health care organizations, patient groups, and industry.
Strategic Salary Support Programs
These programs provide researchers who have reached the stage of independence the opportunity to develop their careers and devote more time and effort to initiating and conducting health research by contributing to their salary.
Strategic Training Programs
These programs provide support and special recognition to students who are pursuing a Master's or PhD degree or for highly qualified candidates at the post-PhD or post-health professional degree stages in strategic priority health research areas. These strategic priority research areas are identified by the Institutes in consultation with
stakeholders from government, health care organization, patient groups, and industry.
Cutting edge discoveries are made at the intersection of research disciplines. CIHR encourages and supports training programs that prepare young researchers to work effectively with a team of colleagues from various disciplines able to focus multiple talents on a single health issue. CIHR took a bold step in 2001 in an effort to improve the health research training environment and increase health research capacity by launching the Strategic Training Initiative in Health Research (STIHR). STIHR provides funding to innovative, interdisciplinary training programs through a grant to a team of mentors, who must use at least 70 per cent of the grant for the support of trainees. STIHR currently supports more than 1,100 trainees at different levels through training centres across the country. The consensus for the need for this type of training is shown by CIHR's decision, along with partner organizations, to relaunch the STIHR funding opportunity in January 2008. This decision was supported by the preliminary findings of the STIHR program evaluation, of which the final report will be completed in March 2008. The evaluation results and case studies will allow CIHR to further improve the program design and framework for ongoing evaluation.
| In 2008-2009, IHDCYH will finalize its Strategic Initiative on Prevention and Treatment of Intentional and Unintentional Injury. This partnership with the Public Health Agency of Canada (PHAC), Alberta Centre for Child, Family and Community Research, AUTO21 Centre of Excellence, Canadian Red Cross, Child Welfare League of Canada, Institut de recherche Robert-Sauvé en santé et sécurité du travail, Ontario Neurotrauma Foundation, SMARTRISK, Transport Canada, WorkSafe BC, IA, IGH and IMHA will fund 5-year Strategic Teams in Applied Injury with funding to start in 2009-2010. |
In addition to providing trainees and independent investigators with salary support, CIHR is committed to helping them develop the skills they require to succeed in the modern research environment. Several Institutes provide their trainees and new investigators interdisciplinary networking opportunities as well as workshops on topics such as building and managing a research team, how to write effective grant proposals, and time management.
On a broader front, CIHR is working with NSERC and SSHRC, the Canadian Association for Graduate Studies (CAGS) and the Society for Teaching and Learning in Higher Education (STLHE) to develop a Statement of Principles on Key Professional Skills for New Researchers. Work on this statement was undertaken because the three granting agencies, universities and employers of highly qualified people have become increasingly aware of the importance of professional skills for new researchers including students, postdoctoral fellows and junior faculty members. Individuals often require, as well as advanced research training, appropriate training in professional skills such as communication, interpersonal skills, project management and leadership in order for them to reach their full potential and maximize their contributions to social and economic benefits for Canadians.
2.2 Program Activity: Research Resources and Collaboration
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $50.9 | $49.5 | $49.5 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 25 | 25 | 25 |
Program Summary
This program enables strengthening the health research community's ability to conduct research by supporting research-enabling activities and resources. This includes engaging in collaborative activities such as networking between researchers, as well as providing and maintaining state-of-the-art tools to conduct research such as new equipment, databases and other specialized
resources. This is achieved through managing and launching competitions and programs to fund grants that give researchers the resources to better undertake their research.
| Expected Results | Indicators |
|---|---|
|
|
| Link to Priority | |
| Strategic Priority #2: Researchers - Develop and sustain Canada's health researchers in vibrant, innovative and stable research environments. | |
Description of Key Programs and Services
CIHR will continue to provide operational support in 2008 to the Canadian Light Source (CLS), a National Facility in Saskatoon that uses synchrotron radiation to conduct high resolution spectrocopic studies on both chemical and biological materials. The CLS synchrotron has numerous potential applications in biological and
medical research, including the determination of molecular structure and the three-dimensional imaging and biological characterization of cells, tissues and whole animals.
CIHR will provide the Structural Genomics Consortium with $2.5 million in 2008-2009 allowing them to provide researchers from Canada and around the world critical structural information for key molecules.
2.3 Program Activity: National and International Partnerships
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $25.2M | $25.2M | $25.2M |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 12 | 12 | 12 |
| In 2008-2009, ICR's Access to Quality Cancer Care initiative will support seven grants. This 5-year partnership with Cancer Care Nova Scotia, Cancer Care Manitoba, IAPH, IGH and IHSPR addresses provincial health priorities with respect to access to cancer care. |
Program Summary
The purpose of this program is to develop strong national and international partnerships, through CIHR's thirteen Institutes, to advance strategic health research priorities, and leverage funding and expertise for research. These partners include health policy-makers at provincial and federal levels of government, the
private sector, and voluntary health organizations from Canada and abroad. This is achieved through funding grants to support Institute activities, managing competitions to fund partnered research projects, and the participation of Canadian scientists in international research collaborations.
| Expected Results | Indicators |
|---|---|
| National and international health research agendas are formulated and implemented, and increased relevance and quantity of research is achieved as a result of strong alliances and partnerships. |
|
| Link to Priority | |
| Strategic Priority #2: Researchers - Develop and sustain Canada's health researchers in vibrant, innovative and stable research environments. | |
Description of Key Programs and Services
CIHR continues to work towards the development of innovative national and international partnerships to increase the quality and quantity of research, as well as to provide operational support, tools and resources to the Institutes to work with their partners in a consistent and transparent manner.
Institute Support Grants
The Institute Support Grant (ISG) program provides funding support to enable Institute activities such as the development of strategic health research priorities and research partnerships with government, health care, patient groups and other stakeholders. These priorities and partnerships, along with Institute-organized conferences, seminars, and workshops, enable
the Institutes to support the growth of the research community and plan and launch their various strategic priority research grants and awards programs.
From its inception, CIHR has provided each of its 13 virtual Institutes with a $1 million support grant annually to facilitate and develop national research networks that link their respective research communities. The Institutes will continue to seek and build upon opportunities to form alliances and networks over the next three years and continue to foster international partnerships which address the research agendas they have established with their communities.
As part of the renewal exercise for the CIHR ISG Program's Treasury Board Terms and Conditions, a detailed review of the virtual Institute funding model was undertaken with results indicating the special requirements and strengths of the virtual model outweighing its challenges.
Partnership Programs
The Partnership program provides grants to enable and support both national and international partnered programs to coordinate health research activities with stakeholders.
Regional Partnerships Program (RPP)
CIHR's Regional Partnerships Program (RPP) promotes health research in provinces that traditionally are not considered as being major centres of health research in Canada. The Program helps build partnerships, and is designed to enable researchers in less populous regions of Canada to meet CIHR's mandate through the creation of
new knowledge and its translation into improved health for Canadians.
Under the RPP, research funding and personnel support applications that are judged to be of high scientific merit through peer review, but are below the funding capacity of CIHR's base budget in CIHR regular competitions, are eligible to receive funding if there is a partner to co-fund the proposal.
The ratio of co-funding is 1 CIHR dollar to 1 partner dollar with an annual maximum of $1 million in CIHR co-funding for each of the four following provinces (Saskatchewan, Nova Scotia, Newfoundland and Manitoba) and $0.2 million each for two provinces (Prince Edward Island and New Brunswick). CIHR's total annual commitment is $4.4 million.
In response to a program evaluation conducted in 2005, the program underwent a redesign in 2006-2007. Items such as minimum eligibility requirements for provincial participation and the competition process were more clearly defined. The redesigned program continues to focus on the needs of the six participating provinces, with its inaugural launch in December 2007. Funding opportunities available through RPP are now launched as priority announcements for each individual program type (eg. Operating grant priority announcement, Fellowship award priority announcement, Doctoral research award priority announcement, etc).
Administratively, each province now has an RPP Advisory Board which manages the program at the provincial level. This management includes pre-screening provincial RPP applications against criteria set by each individual province. Plans for 2008-2009 include convening two RPP National Meetings (one in person and the other via teleconference), in order to discuss issues of the programs of common interest.
Small Health Organizations Partnerships Program (SHOPP)
The mandate of SHOPP is to foster partnership opportunities with small health charities and not-for-profit organizations with modest health research funding capacity by co-funding training and salary awards. The benefit of this program is that CIHR offers partnership opportunities for small health organizations while working to become more in line with the strategic
directions of the Institutes.
An external evaluation of the program was completed in 2007-2008 and demonstrated that this program is meeting the overall objectives of SHOPP: it is benefiting its intended target groups which are small health charities, graduate students/'early career stage' researchers and is providing benefits to the partnering organizations and researchers. One of the main challenges is finding researchers interested in very limited areas of research. Plans for 2008-2009 include developing communication strategies to address this challenge and working with partners to integrate the researchers funded by SHOPP into their organizations.
2.4 Program Activity: Ethical, Legal and Social Issues (ELSI)
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $2.9 | $3.0 | $3.0 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 2 | 2 | 2 |
Program Summary
The purpose of this program is to promote and assist the conduct of research on ethical, legal and social issues pertaining to health and health research, and the application of ethical principles to health research. This is achieved through managing competitions to fund grants for research on health-related ethical, legal and social issues, and conducting consultations to foster
dialogue and greater engagement.
| Expected Results | Indicators |
|---|---|
| Health research conducted more ethically as a result of effective funding programs. |
|
| Link to Priority | |
| Strategic Priority #2: Researchers - Develop and sustain Canada's health researchers in vibrant, innovative and stable research environments. | |
Description of Key Programs and Services
CIHR funds research on ethical, legal and social issues (ELSI) related to health and health research. In addition, CIHR engages in inclusive dialogue across sectors, disciplines and communities and pursues public engagement to improve knowledge and understanding of
ELSI in the context of health and health research.
Promoting Research on ELSI Related to Health and Health Research
CIHR and multiple partners promote research on cross-cutting ELSI as an integral part of the national health research agenda. In 2008-2009, CIHR will provide $2.0 million to support strategic initiatives in this area. CIHR will also support additional research in this area through its open funding
competitions, and a number of Institute-sponsored initiatives. By providing this fund, CIHR has signaled a commitment to build capacity among investigators who are poised to conduct research and translate new knowledge in strategically important research areas related to ELSI.
The Ethics Office has committed the following to new funding opportunities in 2008-2009:
- $0.225 million to an Operating Grant Priority Announcement, to support excellent applications for research projects in ethics on any relevant research questions within the domains of biomedical sciences, clinical sciences, health services and policy, and population and public health; and conceptual and/or empirical research addressing ethical challenges relevant to CIHR's mandate; and
- $0.225 million to a Catalyst Grant RFA to encourage applications from new investigators entering the field of ethics of health and health research, and mid-career investigators interested in transitioning into this field.
Contributing to Broader Health Policy Debate
CIHR is committed to promoting health research that meets the highest international standards of excellence and ethics. CIHR works collaboratively with many partners to develop the highest ethical standards for health research and to see to their application in practice. This includes funding the Canadian Council on Animal Care, in partnership
with the NSERC, and the National Council on Ethics of Human Research in partnership with Health Canada. In addition, in 2008-2009, CIHR will continue to build on the work done to date in relevant public policy areas:
- The launch of the initial implementation phase for CIHR's Best Practices for Protecting Privacy in Health Research, along with ongoing contribution to policy and legislative initiatives at the federal and national level relevant to health research and privacy issues;
- The implementation of a national policy respecting the appropriate use of placebos in randomized controlled trials; and
- The implementation of CIHR Guidelines for Health Involving Aboriginal People.
In delivering results related to Strategic Outcome #2, People and Research Capacity, CIHR is facing the following risks and challenges:
- As noted earlier, many high quality applications cannot be funded. Should this situation not improve, the viability of a career in health research could be undermined and CIHR's ability to achieve its mandate to foster excellence could be compromised; and
- Many research environments remain traditional and do not offer students and researchers the opportunity to learn from colleagues in different disciplines or with expertise in other relevant areas including knowledge translation and ethics.
CIHR will address these challenges and mitigate these risks with the following strategies:
- Offering prestigious awards and scholarships to the very best students and researchers as a motivating example of excellence;
- Stimulating the curiosity of young people and their interest in a rewarding career in science and health research;
- Exploring new programming that better supports researchers through-out their careers, particularly during the initial stages; and
- Continuing to help foster novel learning environments that are multidisciplinary and hold the promise of accelerating training outcomes.
3. Strategic Outcome: Knowledge Translation and Commercialization
CIHR's Strategic Outcome 3.0 ensures that:
health research is translated and adopted into practice, programs and policies that offer more effective health services and products, a strengthened health care system, and the improved health of Canadians
| Expected Result | Indicators |
|---|---|
| Translation and use of health research takes place as a result of effective funding programs, which can lead to benefits to Canadians. |
|
By supporting knowledge translation (KT), CIHR aims to accelerate the transformation of research results into health benefits for Canadians and an improved health care system. This includes funding knowledge translation research, synthesis, dissemination, exchange and application activities and building knowledge translation networks. KT at CIHR also involves and includes helping to move promising new research breakthroughs toward potential commercial applications.
Throughout 2008-2009 and beyond, CIHR will facilitate the use of relevant health research to improve practice, programs and policies for a productive health system, and to stimulate economic development through discovery and innovation.
3.1 Program Activity: Knowledge Translation of Health Research
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $40.8 | $41.1 | $41.1 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 20 | 20 | 20 |
| In 2008-2009, INMD will finalize its Intervention Research on Healthy Living and Chronic Disease Prevention initiative. This 3-year partnership with the Health Canada's First Nations Inuit Health Branch support prompt initiation of intervention and evaluation research on programs, events, and/or policy changes that have been initiated and with a potential impact on healthy living and chronic disease prevention. Funding of these grants is expected to begin in 2009-2010. |
Program Summary
The purpose of this program is to enable the effective dissemination and exchange of health research knowledge, and the application of health research results and discoveries to lead to improvements in the Canadian health system and overall health of Canadians. This is achieved through managing competitions and programs to fund grants for translating health research discoveries into
new or more effective health policy or practice, and for building increased knowledge translation capacity in Canada's health research community.
| Expected Results | Indicators |
|---|---|
| Health research is translated more effectively as result of funding programs. |
|
| Link to Priority | |
| Strategic Priority #3: Knowledge Translation - Catalyze health innovation in order to strengthen health and the health care system and contribute to the growth of Canada's economy | |
| In 2008-2009, IMHA will be funding grants in their Community Alliance in Health Research and Knowledge Exchange on Pain initiative. This 5-year collaboration with AstraZeneca and INMHA will fund grants to engage active partnerships between research teams and community organizations which will foster high quality research and knowledge translation exchange in research on Pain. |
Description of Key Programs and Services
CIHR supports dissemination and use of research knowledge through funding research on knowledge translation and developing tools, programs and strategies.
Knowledge Translation Programs
The Knowledge Translation program provides grants for effective research on knowledge translation, and to enable the effective dissemination, exchange, synthesis and application of health research results that will lead to improvements in the Canadian health system and improved health for Canadians.
Supporting Knowledge Translation
CIHR supports two types of KT, end of grant KT and integrated KT. End of grant KT allows the researcher to develop and implement a plan for making knowledge users aware of the knowledge generated through a research project. End of grant activities could include presenting
at conferences or publishing in peer-reviewed journals. Integrated KT is an integral way of achieving knowledge exchange and application. Potential knowledge users are engaged as partners in the entire research process, from identifying research questions to interpreting, disseminating and applying the research findings.
In 2008-2009, given the increased demand from the researcher community, CIHR will continue to expand its investments in KT through initiatives such as the Knowledge Synthesis Request for Applications (RFA), the annual Knowledge to Action RFA, the Proof of Principle (POP) program, Partnerships for Health System Improvement (PHSI), and the Meeting, Planning and Dissemination (MPD) Grants Program.
KT Strategy
An integral part of CIHR's mandate, KT is about moving knowledge to action: turning the knowledge gained through health research into improved health for Canadians, more effective services and products, and a strengthened health system. CIHR's KT Strategy, including KT focused on
commercialization, is designed to further expand and increase its ongoing efforts in this area. The strategy is a multi-year plan and identifies planned activities in the following four areas:
| In 2008-2009, IHSPR will begin funding the Health Services and Policy Research Chair Program. This partnership with Health Canada, the Canadian Health Services Research Foundation, the Ontario Ministry of Health and Long Term Care, the Canada Health Infoway and the Canadian Patient Safety Institute (partner funding representing 43% of all funding) will provide 5-year funding across IHSPR's strategic themes (Access to Appropriate Care across the Continuum, Drug Policy and Health Information) as well as the themes from the Listening From Direction III consultation exercise (http://www.cihr-irsc.gc.ca/e/20461.html). |
- Supporting research on KT concepts and processes;
- Contributing to building networks of researchers and end-users;
- Improving capability to support KT research at CIHR and with partners; and
- Supporting and recognizing KT excellence.
KT efforts at CIHR aim to fund KT research, facilitate partnerships that accelerate KT, and evaluate KT efforts by assessing impact. To strengthen health and the health care system and contribute to the growth of Canada's economy, CIHR will build on its first KT strategic plan with revisions planned for 2008 and beyond incorporated into the Blueprint II strategic planning process. Throughout 2008-2009, CIHR will support KT research and KT researchers through, for example, initiatives that fund teams of researchers and decision makers working together to explore new ways of turning knowledge into improved health and health care for Canadians, and initiatives that support knowledge synthesis, exchange and application, including training in these areas. CIHR will aim to build on its KT efforts by continuing to support both end of grant and integrated KT as fundamental components of strategic initiatives, including those focused on commercialization. CIHR's 13 Institutes play an important role in supporting and promoting KT at CIHR.
| In 2008-2009, IPPH will facilitate the further implementation of a partnership with the Public Health Agency of Canada (PHAC), other CIHR Institutes, the Centre de recherche en prévention de l'obesité and the Heart and Stroke Foundation to fund at least 11 Applied Public Health Chairs. This 5-year initiative will lead to increased national capacity for effective intervention research, application of research evidence of relevance to the public health system, mentoring and education. |
KT Training and Capacity Building
In 2008-2009 CIHR will support a variety of initiatives for KT training and capacity building, such as the KT Doctoral Research Awards, Fellowships and New Investigator Awards, Health Research Communications Award (HRCA), the KT Assessment Project, the KT Handbook,
KT training requirements in the Strategic Training Initiative in Health Research (STIHR), KT Synthesis Training Modules, Building a network of KT trainees, and Commercialization Programs.
CIHR KT Policies
In 2008-2009, CIHR will develop, implement and evaluate a number of projects focused on KT related policies, such as the open access policy, randomized control trials-related policies, commercialization policy, and merit review policy.
Networks of Centres of Excellence Grants Program
The Networks of Centres of Excellence (NCE) Grants program provides funds to support the best NCE applications in areas of health research. NCE's are nation-wide, multidisciplinary and multi-sectoral partnerships that connect excellent research with industrial know-how and strategic
investment aimed at turning Canadian research and entrepreneurial talent into economic and social benefits for Canada.
CIHR will continue to administer $27.5 million annually towards the NCE program, in collaboration with Industry Canada and the other two federal granting councils. The networks are unique partnerships among universities, industry, government and not-for-profit organizations aimed at turning Canadian research and entrepreneurial talent into economic and social benefits for all Canadians. These nation-wide, multidisciplinary and multi-sectoral research partnerships connect excellent research with industry know-how and strategic investment.
The NCE secretariat is responsible for three new major funding programs: the Centres of Excellence for Commercialization and Research (CECR), the Business-led NCEs, and the Industrial R&D Internship program.
3.2 Program Activity: Commercialization of Health Research
Financial Resources (in millions)
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| $27.5 | $26.7 | $26.7 |
Human Resources
| 2008-2009 | 2009-2010 | 2010-2011 |
|---|---|---|
| 14 | 13 | 13 |
Program Summary
The purpose of this program is to encourage innovation and facilitate the commercialization of health research in Canada into new health products and services. This is achieved through managing competitions to fund grants for supporting the commercialization of health research, in partnership with the private sector, and for building increased commercialization capacity in Canada's
health research community.
| Expected Results | Indicators |
|---|---|
|
|
| Link to Priority | |
| Strategic Priority #3: Knowledge Translation - Catalyze health innovation in order to strengthen health and the health care system and contribute to the growth of Canada's economy | |
Description of Key Programs and Services
Commercialization is an integral part of CIHR's KT mandate. CIHR provides various funding programs in support of implementation of its commercialization and innovation strategy, which are focused in four areas: research, talent, capital and linkages. CIHR's strategy focuses on the
early stages of commercialization, where there is a growing gap between a promising initial concept and its exploitation for health and economic advantage. CIHR's initiatives encourage and better enable universities and teaching hospitals to interact with partners from the public and private sectors that do late-stage development and ultimately deliver the benefits
of health research.
In 2008-2009, CIHR intends to continue to expand its investment in commercialization through the following activities.
Mobilizing Research
Through this strategy, CIHR will recruit the private sector and implement new national platforms and initiatives to support clinical research, technology and drug development programs. The clinical research related programs will develop centres, platforms and expertise in specialized areas of clinical research. Technology programs and drug development programs will
promote and facilitate the advancement of new research tools and techniques, and identify promising drug compounds discovered in academia respectively.
Proof of Principle (POP) Program
This is CIHR's core commercialization program offering 12-month grants to complete proof of principle research. Each application is comprised of, and rated to, it's scientific/research and commercialization merit. Funding for drug development, currently $0.5 million a year, is currently delivered as a priority announcement through the POP program
(launched in December each year with two competition cycles).
Science to Business (S2B) Program
The program enables recent PhD graduates to get a health/biotech MBA. The applicants are Canadian business schools. Historically, $0.5 million a year was allocated to the program; however due to its success, the budget has increased to $1.0 million for 2007-2008 onward. S2B is typically launched once a year.
CIHR's Innovation and Industry Programs
CIHR's Innovation and Industry Programs are designed to help the academic community interact with Canadian companies with an interest in health research and development. The programs promote a variety of peer-reviewed research and training projects jointly funded by Canadian companies and CIHR. The research undertaken at eligible institutions
under the leadership of a principal applicant who is affiliated with the institution is based on research excellence focused on ultimately improving the quality of health of Canadians. The two main arms to CIHR's Innovation and Industry Programs include the CIHR/Rx&D
Collaborative Research Program and the CIHR/Small and Medium-Sized Enterprise (SME) Research Program.
In alignment with the Clinical Research Initiative, the CIHR/Rx&D Collaborative Research Program, with Canada's research based pharmaceutical companies, is enhancing research opportunities in Canada's research institutions and development of health research personnel stimulating jobs and growth in the Canadian economy.
Over the past eight years, the program has invested over $150 million3 ($40 million of which was contributed by CIHR) in valuable research projects at universities and teaching hospitals across Canada, making this public-private research agreement one of the largest in the country. The objective of the program will continue to focus on building clinical research support through personnel awards such as CIHR-Rx&D Research Chairs and operating support programs, including research grants and clinical trials.
Furthermore, the program will encourage the sharing of best practices in clinical research, leading to better training of investigators and more comprehensive clinical trials. Activities such as this reflect an ongoing commitment to research and the creation of new and innovative ideas, ideas critical to improved health and Canada's competitiveness in the global knowledge-based economy.
The CIHR/Small and Medium-Sized Enterprise (SME) Research Program with Canada's developing biopharmaceutical community encourages and strengthens the health research programs of start-ups, university spin-offs, and SMEs, and strengthens intellectual property (IP) portfolios in partnership with Canadian biotech companies. Currently this program offers multiple CIHR funding tools (training and salary awards, operating grants and RCTs).
Conscious of the issues that may arise from the academic/industry interface and the potential for ethical conflict between profit and the public good, CIHR will be leading an industry/university effort to review and propose standards for ethical conduct of projects in the commercialization and innovation arena.
Partnerships are not limited to the private sector. Successful development of heath innovations requires multidisciplinary collaborations. CIHR will further develop its successful collaborations with federal and provincial organizations (such as the CIHR/NSERC Collaborative Health Research Program, and medical device consortiums respectively). CIHR and NSERC funding for this program will double in 2008-2009 based on the importance of collaborative health research.
3. These figures represent only partner funds administered by CIHR. As in-kind partner contributions can not accurately be validated and that partner funds not administered by CIHR are not included, partner contributions are likely understated.
In achieving results in Strategic Outcome #3, Knowledge Translation and Commercialization, CIHR is facing the following risks and challenges:
- The need to raise awareness and understanding among all researchers of good practices for integrated and end-of-grant knowledge translation;
- The need to work effectively with a multitude of players involved in the complex and uncertain process of innovation and knowledge application;
- The need to better integrate potential knowledge users (industry, policy makers, clinicians, etc) into the research process, to optimize the relevance of the research being conducted, and
- The relative shortage of Canadians with specialized skills and experience in knowledge translation.
The risk of inadequately addressing these challenges is that Canadians would not benefit as fully or as quickly as they should from the new knowledge produced through research.
CIHR is responding to these challenges and risks through:
- Integrating knowledge translation activities into all of its programs. For example, increasingly CIHR will make plans for dissemination of research results a requirement as part of the application process for grants. Under CIHR's new Open Access policy, researchers will be required to ensure their findings are readily accessible to all interested parties free of charge via the internet;
- Funding 'synthesis' research that is not focused as much on original research but on summarizing findings across a body of research for practical application by other researchers and users of research including clinicians and public policy makers;
- Supporting research advancing the science of knowledge translation; and
- Organizing symposia to bring researchers, industry, health care professionals and policy makers together.
