Common menu bar links
Breadcrumb Trail
ARCHIVED - Patented Medicine Prices Review Board
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Chairperson's Message
I am pleased to present the 2008-2009 Departmental Performance Report for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act. Its mandate is two-fold: Regulatory - to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and Reporting - to report on pharmaceutical trends of all medicines, and on R&D spending by pharmaceutical patentees. The PMPRB contributes to the broader objective of improving the health of Canadians by protecting consumers and Canadian health care from excessive patented drug prices and contributing to informed drug policy decision-making.
In 2008 there were 1,260 patented drug products under the Board's jurisdiction, including 78 new drugs reported that year.
The PMPRB’s capacity to carry out its regulatory mandate is dependent on relevant and effective Excessive Price Guidelines (Guidelines) and on the ability to conduct hearings, when required.
In 2005, the Board initiated a process to review its Excessive Price Guidelines, including consulting with key stakeholders as required by the Patent Act. Significant progress occurred in 2008-2009, with the release of two rounds of Notice and Comment on draft revised Guidelines. The final round of consultations on the Guidelines took place in late spring of 2009. The revised Guidelines were released in June 2009, with an implementation date of January 1, 2010. Outreach educational sessions have already commenced, and an evaluation of the impact of the changes made will be undertaken on an ongoing basis.
The Board issued four Notices of Hearing in 2008, into the matters of Apotex and ratiopharm Inc., regarding failure to file, and into the prices of the medicines Apo-Salvent CFC Free and ratio-Salbutamol HFA. On March 16, 2009, the Board issued a Notice of Hearing into the matter of Amgen Canada Inc. and the price of the medicine Neulasta.
In order to enable the Board to meet the resource demands of effectively carrying out its regulatory mandate, on September 4, 2008, the PMPRB received the approval of Treasury Board for additional resources. These new resources will enable the PMPRB to effectively implement and monitor the revised Guidelines; provide educational outreach to patentees and other stakeholders; carry out ongoing price reviews and investigations within reasonable timelines; and hold timely hearings, as required.
In 2008-2009, the PMPRB continued to work with the advice of participating federal, provincial and territorial drug plans, and in collaboration with the Canadian Institute for Health Information (CIHI), to produce analyses and reports under the National Prescription Drug Utilization Information System (NPDUIS).
The PMPRB remains committed to predictability, fairness and transparency in the fulfilment of its regulatory and reporting responsibilities, to ongoing engagement of its key stakeholders, and to continual monitoring of developments within the larger pharmaceutical environment that may relate to, or impact upon, the conduct of the Board’s mandate.
Brien G. Benoit, MD
Chairperson
Section 1 — Board Overview
Raison d'être
The Patented Medicine Prices Review Board (PMPRB) has a dual role:
Regulatory — To ensure that prices charged by patentees for patented medicines sold in Canada are not excessive.
Reporting — To report on pharmaceutical trends of all medicines, and on R&D spending by pharmaceutical patentees.
Responsibilities
The PMPRB is an independent, quasi-judicial body created by Parliament as a result of revisions to the Patent Act (Act) in 1987 (Bill C-22). The Act was further amended in 1993 (Bill C-91). The revisions were intended to balance changes within the government's overall pharmaceutical patent policy regime.
Regulatory Role
The PMPRB is responsible for regulating the prices that patentees charge for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, to ensure that they are not excessive. The PMPRB regulates the price of each patented drug product (each strength of an individual, final dosage form of a patented drug). This is normally the level at which Health Canada assigns a Drug Identification Number (DIN) as part of the Notice of Compliance process.
The Federal Court of Appeal articulated the legal requirement as to when a patent will "pertain" to the medicine. In this regard, the Court established the "merest slender thread" requirement, which is wide in scope. The Board's jurisdiction is not limited to drug products for which the patent is on the active ingredient. Rather, the Board's jurisdiction also covers drugs for which the patents relate to, but are not limited to, the processes of manufacture, the delivery system, dosage form, indication/use, and/or any formulation. Patented drugs are not limited to brand name products. A number of generic companies fall under the Board's jurisdiction by virtue of being licensees, authorized to sell the same drug product as the brand company is selling, or because of their own patents (e.g. related to processing or manufacturing).
The PMPRB has no authority to regulate the prices of non-patented drugs and does not have jurisdiction over prices subsequently charged by wholesalers or retailers or over pharmacists� professional fees. Also, matters such as whether drugs are reimbursed by public drug plans, distribution channels and prescribing are outside the purview of the PMPRB.
Under the Patented Medicines Regulations, patentees are required to inform the PMPRB of their intention to sell a new patented drug product. Upon the sale of such a patented drug product, patentees are required to file price and sales information for the first day's sales and, thereafter, twice a year for six month sales for each strength of each dosage form of each patented drug product sold in Canada for price review/regulation purposes, for the duration of the patent.
Although patentees are not required to obtain the PMPRB's approval of the price of a patented drug before it is sold, they are required to comply with the Act to ensure that prices of patented drug products sold in Canada are not excessive. In the event that the Board finds, after a public hearing, that a price is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting Role
The PMPRB reports annually to Parliament, through the Minister of Health, on its activities, on pharmaceutical trends relating to all medicines, and on the R&D spending by pharmaceutical patentees. In addition to these reporting responsibilities, under section 90 of the Act the Minister of Health has the authority to direct the PMPRB to inquire into any matter. Under this provision, the Minister has directed the Board to undertake two initiatives: the National Prescription Drug Utilization Information System (NPDUIS), and monitoring and reporting on Non-Patented Prescription Drug Prices (NPPDP).
National Prescription Drug Utilization Information System (NPDUIS)
Since 2001, pursuant to an agreement by federal, provincial and territorial Ministers of Health, the PMPRB has been conducting research under the NPDUIS. The purpose of the NPDUIS is to provide critical analyses of price, utilization and cost trends so that Canada�s health system has more comprehensive and accurate information on how prescription drugs are being used and on sources of cost drivers.
Non-Patented Prescription Drug Prices (NPPDP)
In 2005, the Minister of Health, on behalf of federal, provincial and territorial Ministers of Health, directed the PMPRB to monitor and report on non-patented prescription drug prices. This function is aimed at providing a centralized credible source of information on non-patented prescription drug prices. Since April 2008, NPPDP studies are conducted under the umbrella of the NPDUIS.
Strategic Outcome and Program Activity Architecture (PAA)
The PMPRB has one Strategic Outcome (SO) and two program activities (PAs) - representing its regulatory and reporting roles - which are illustrated in the following chart:
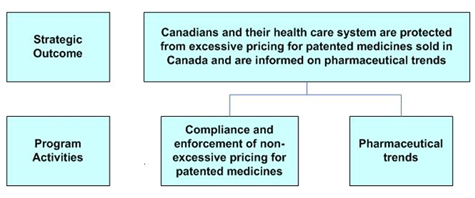
Compliance and enforcement of non-excessive pricing for patented medicines
The PMPRB is responsible for regulating the prices that patentees charge for patented drugs sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use. Through this program activity, the PMPRB reviews the prices that patentees charge for patented drugs, based on the price review factors in the Patent Act, to ensure that these prices are not excessive. In the event that the Board finds, following a public hearing, that a price is excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received as a result of excessive prices.
Pharmaceutical trends
Through this program activity, the PMPRB provides analysis of pharmaceutical price trends and research and development spending by pharmaceutical patentees. It also provides critical analyses of price, utilization and cost trends for prescription drugs, and information on non-patented prescription drug prices. The PMPRB reports on this information and on its price review and enforcement activities as they relate to excessive pricing for patented medicines, both annually to Parliament, through the Minister of Health, and through special published studies.
Performance Summary
| Planned Spending | Total Authorities | Actual Spending |
|---|---|---|
| $5,842.0 | $11,159.6 | $8,050.2 |
| Planned | Actual | Difference |
|---|---|---|
| 46.0 | 49.0 | (3.0) |
On September 4, 2008, the PMPRB received the approval of Treasury Board for additional resources to enable the Board to effectively carry out its regulatory mandate. As a result, a significant portion ($4.7M) of the total spending authority was pending until the approval of Supplementary Estimates (B) in February 2009. For most of the year, the Board's focus was primarily to ensure that critical activities related to the review of its Excessive Price Guidelines and ongoing price reviews, investigations and hearings were carried out.
With respect to FTEs, the PMPRB began anticipatory staffing of critical positions after approval of the funding submission on September 4, 2008, which did not conclude until late in the fiscal year or the next year.
Performance Summary Table
| Strategic Outcome: Canadians and their health care system are protected from excessive pricing for patented medicines sold in Canada and are informed on pharmaceutical trends. | ||
|---|---|---|
| Performance Indicators | Targets | 2008-2009 Performance |
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. | Canada's prices on average are at or below the median of international prices. | In 2008, Canada's prices were on average slightly higher than the median of international prices. Further information is available in the PMPRB's Annual Report 2008, Figure 12, at http://www.pmprb-cepmb.gc.ca/english/view.asp?x=91&mp=68 |
| Program Activity | 2007-2008 Actual Spending | 2008-2009 | Alignment to Government of Canada Outcomes | |||
|---|---|---|---|---|---|---|
| Main Estimates | Planned Spending | Total Authorities | Actual Spending | |||
| PA 1: Compliance and enforcement of non-excessive pricing for patented medicines | $5,706.5 | $3,194.0 | $3,194.0 | $7,955.0 | $5,263.1 | Healthy Canadians: by ensuring that prices of patented drug products are not excessive. |
| PA 2: Pharmaceutical trends | $1,725.9 | $2,648.0 | $2,648.0 | $3,204.6 | $2,787.1 | Healthy Canadians:
|
| Total | $7,432.4 | $5,842.0 | $5,842.0 | $11,159.6 | $8,050.2 | |
Of the total spending authorities for PA 1, $2.2 million was provided in a Special Purpose Allotment (SPA) dedicated to expenditures on hearing costs. Of this SPA, $1.4M lapsed in 2008-2009 due to the fact that some ongoing hearings were settled when patentees unexpectedly submitted Voluntary Compliance Undertakings (VCUs), while other matters that were anticipated to be recommended to move to a hearing were delayed as a result of further discussions with patentees. The remaining lapse (in PA 1 and PA 2) was primarily due to delays in staffing new and vacant positions.
Contribution of Priorities to Strategic Outcome
| Operational Priorities | Type | Status | Linkages to Strategic Outcome1 |
|---|---|---|---|
| Guidelines Review | Ongoing | Mostly met The final round of consultations was initiated in March 2009. The revised Guidelines were released on June 9, 2009. The implementation is scheduled for January 1, 2010. |
The revised Guidelines provide greater transparency, fairness and predictability for patentees in setting prices for patented drug products that will not be considered excessive. The increased clarity of the Guidelines and flexibility in unique situations is expected to facilitate patentees' compliance with the Act. |
| Voluntary compliance with Guidelines encouraged | Previously committed to | Mostly met 87% of patented drugs were within the Board's Guidelines. |
Pricing by patentees of patented drug products at levels that are not excessive contributes to the overall affordability of patented drugs for Canadians and the health care system. |
| Patented drug prices reviewed in a timely manner | Ongoing | Successfully met 95% of drug products first sold in 2008 were reviewed in that year for conformity with the Guidelines. 99% of existing drug products in 2008 were also reviewed. |
Timely review of patented drug prices to ensure they are not excessive is key to protecting consumer interests. |
| Management Priorities | Type | Status | Linkages to Strategic Outcome |
|---|---|---|---|
| Seek approval to increase the Board's permanent funding to reflect ongoing pressures and operational needs. | New | Successfully met On September 4, 2008, Treasury Board gave the PMPRB authority to include $4.7 million (excluding EBP) in Supplementary Estimates (B), and to increase reference levels in Vote 35 (Program expenditures) by $5.6 million for 2009-2010. |
Supplemental funding has enabled the PMPRB to meet the resource demands of effectively carrying out its regulatory mandate to ensure that the prices of patented drugs sold in Canada are not excessive, which is in the interest of Canadians. |
Risk Analysis
The PMPRB had previously identified risks of funding shortfalls under its regulatory mandate stemming from an unprecedented number of investigations into excessive drug prices and anticipated hearings, and the need to update and modernize the Board's Excessive Price Guidelines to ensure they remain relevant and effective.
In 2008-2009, the PMPRB obtained permanent additional funding in the amount of $4.7 million (excluding EBP). This has mitigated the risk that the PMPRB will not have sufficient resources to carry out its regulatory mandate, but it still faces challenges with respect to the staffing of new positions.
In terms of the Guidelines review, the organization was challenged by the significance of, and wide-ranging views of stakeholders on the issues being addressed. However, comprehensive, iterative consultations with all stakeholders, including the pharmaceutical industry, governments and consumers/patient interest groups and others, have resulted in revised Guidelines that are clearer, more transparent and more relevant to the modern pharmaceutical environment.
The Board released its revised Guidelines in June 2009, with an implementation date of January 1, 2010. Strategies to mitigate risks related to the operationalization of the revised Guidelines will include extensive communication with patentees, and other stakeholders, via various briefing/information sessions and presentations.
In 2008-2009, the Board's decisions and Orders faced a number of Judicial Reviews.
A Hearing Panel of the Board issued its decision and reasons in the matter of Teva Neuroscience and the new medicine Copaxone on February 25, 2008, and its Order on May 12, 2008. The Respondent filed an application for Judicial Review with the Federal Court. A hearing date has not yet been scheduled.
In a decision dated January 21, 2008, the Board ruled that it had jurisdiction over the price of Thalomid, as provided to Canadian patients under Health Canada's Special Access Programme. Celgene Corporation filed an application for Judicial Review arguing that the Board does not have any jurisdiction over the pricing of Thalomid. The Federal Court issued a decision on March 17, 2009 dismissing the Board's decision. The Attorney General of Canada has filed a Notice of Appeal with the Federal Court of Appeal. The matter has not yet been scheduled for hearing.
Following the Board's release of a Communiqué in August 2008, dealing with the mandatory reporting of benefits as per the Patented Medicines Regulations, Rx&D et al and Pfizer Canada Inc. commenced Judicial Reviews. The Federal Court heard these matters in June 2009 and issued a decision on July 10, 2009, overturning the Board's requirement that patentees include third party payments in the calculation of net average prices. A decision by the Attorney General of Canada on whether to appeal the matter is pending.
Patentees have the legal right to file Judicial Reviews of the Board's decisions, so this is an ongoing risk. However, as a result of Federal Court decisions, matters pertaining to the Board's interpretation of its statutory mandate are being reviewed and clarified, which ultimately assists both the Board and patentees.
Expenditure Profile
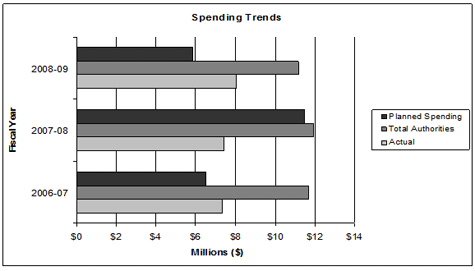
In recognition of increasing workload pressures, the PMPRB was provided program integrity funding in both 2006-2007 and 2007-2008.
In anticipation of the sunsetting of the temporary program integrity funding at the end of March 2008, the PMPRB prepared a business case which identified ongoing pressures and resource needs. In September 2008, Treasury Board gave the PMPRB the authority to include an item in the Supplementary Estimates (B) in the amount of $4.7 million (excluding EBP) (in addition to the core A-base of $5.8 million), to enable the PMPRB to meet its workload pressures and continue ongoing initiatives related to the delivery of its mandate.
As part of these increased resources, authority was given to increase the Special Purpose Allotment (SPA) to conduct Public Hearings, in Vote 35 (Program expenditures) by $1.9 million in 2008-2009. The SPA can only be used to cover the costs of public hearings.
In 2008-2009, the PMPRB is reporting expenditures of $8.1 million.
Overall, the PMPRB lapsed $1.4 million from the Special Purpose Allotment, primarily because some ongoing hearings unexpectedly ended when the patentee submitted a Voluntary Compliance Undertaking to reduce the drug product's price and offset excess revenue, and other matters that were expected to be referred to the Chairperson to issue a Notice of Hearing were delayed. It is difficult for the PMPRB to accurately predict these hearing costs from year to year, given the uncertainty in the number of hearings that will take place and the fact that costs vary for different hearing phases (e.g. the pre-hearing phase is less costly than the hearing on the merits which may involve numerous witnesses).
Another major source of the budget lapse was the uncertain timing of the receipt of incremental resources and the ongoing challenge of small agencies like the PMPRB in attracting and retaining human resources.
Voted and Statutory Items ($ thousands)
| Vote # or Statutory Item (S) | Truncated Vote or Statutory Wording | 2006-2007 Actual Spending | 2007-2008 Actual Spending | 2008-2009 Main Estimates | 2008-2009 Actual Spending |
|---|---|---|---|---|---|
| 35 | Program expenditures | $6,742.5 | $6,722.5 | $5,211.0 | $7,385.9 |
| (S) | Contributions to employee benefit plans | $622.8 | $709.9 | $631.0 | $664.3 |
| Total PMPRB | $7,365.3 | $7,432.4 | $5,742.0 | $8,050.2 | |
The increase in expenditures between the 2007-2008 and 2008-2009 reporting periods relates in large part to a one-time major retrofit of the PMPRB facilities and significant enhancements to the mission-critical PMPRB database, both of which were provided for in the supplemental funding provided through Supplementary Estimates (B). These increases were partially offset by vacancies in several existing and new positions.
