Common menu bar links
Breadcrumb Trail
ARCHIVED - Hazardous Materials Information Review Commission Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Section I – Overview
President’s Message
As the newly appointed President and Chief Executive Officer of the Hazardous Materials Information Review Commission (HMIRC), I am pleased to submit to Parliament, and to Canadians, the Commission’s 2008–2009 Report on Plans and Priorities. It focuses on the Commission’s fundamental commitment to the health and safety of Canadian workers while supporting the chemical industry’s competitiveness in the Canadian market. Our vision continues as a quasi-judicial organization, that takes pride in making sound, evidence-based decisions and seeks creative and progressive approaches to fulfilling its mandate.
HMIRC operates within the context of the Workplace Hazardous Materials Information System (WHMIS) and is accountable to Parliament through the Minister of Health. Under WHMIS and the Hazardous Materials Information Review Act, the Commission is mandated to achieve a balance between the right of workers to know about the hazardous materials they deal with in the workplace and the right of industry to protect confidential business information. Through our compliance efforts, the Commission continues to be an important health and safety advocate for workers as well as a strategic partner to industry, helping to safeguard trade secrets that help companies compete in the marketplace. Over the past decade, the value of the trade secrets submitted to the Commission for protection totaled close to $3 billion.
Over the next three years, the Commission will focus on its core mandate through four priorities:
First, the Commission will continue streamlining its Claims Exemption Process – our key program activity. The objective is that clients can market their products without delay, claims are adjudicated expeditiously and most importantly that employers and workers have access to complete and accurate information on how to handle hazardous products in the workplace. These procedural improvements will be complemented by the development and implementation of an aggressive human resources strategy to address our past challenges of recruiting and retaining scientific staff. The initiatives identified in this plan will help to maintain a steady workforce and keep claims processing at a level that meets the annual demand.
However, over the past years, HMIRC has experienced a significant increase in both the number and complexity of claims. This, coupled with the chronic shortage of qualified scientific personnel has resulted in a growing backlog of claims to be processed. The claims backlog causes considerable delay in issuing orders for compliance to claimants relative to the mostly non-compliant material safety data sheets awaiting review. This, in turn, places Canadian workers at greater risk.
So, we are challenged on two fronts: building and maintaining a skilled and knowledgeable workforce in an intensely competitive employment market, and the increasing backlog of claims for exemption. HMIRC requires additional resources to meet these challenges and is actively exploring options to augment the current resource base.
Enhancing management excellence remains an ongoing priority at HMIRC. By continuing to strengthen strategic partnerships with other departments and within the Health Portfolio, the Commission is able to access interdepartmental resources and expertise. Our emphasis on sound resource management and effective decision-making is entirely consistent with the Government of Canada’s Management Accountability Framework.
I am proud to announce that the legislative amendments sought in Bill S-2, An Act to Amend the Hazardous Materials Information Review Act, were given Royal Assent on March 29, 2007, completing the Commission’s renewal process. The effect of these amendments will be to reduce the time required to review claims for exemption, speed up the correction of information needed by workers to handle hazardous products safely and streamline the appeals process. Consequential amendments to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations are anticipated to be completed by Fall 2008, at which point both legislative and regulatory changes will take effect.
Finally, on the international front, this agency plays a key role in helping to foster progress towards the goal of the United Nations initiative, Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Their objective is to enhance the protection of human health and the environment by providing an internationally comprehensible system for hazard communication. HMIRC provides unique expertise and knowledge given its position as Canada’s only federal/provincial/territorial agency that manages trade secret protection within WHMIS.
I look forward, in my new capacity as President & CEO, to successfully meeting the challenges this planning period will present. I consider these challenges and strategic priorities as an opportunity for dialogue with the Commission’s partners and stakeholders to reaffirm our commitment to balancing industry confidentiality with workers’ health and safety. I can assure our stakeholders and the HMIRC Council of Governors, as well as my staff, that this Commission will remain dedicated to providing high quality service to claimants as we continue to make an important contribution to Canadian workplace health and safety.
Sharon A. Watts
President and Chief Executive Officer
Management Representation Statement
I submit for tabling in Parliament, the 2008–2009 Report on Plans and Priorities (RPP) for the Hazardous Materials Information Review Commission.
This document has been prepared based on the reporting principles contained in Guide for the Preparation of Part III of the 2008–09 Estimates: Reports on Plans and Priorities and Departmental Performance Reports:
- It adheres to the specific reporting requirements outlined in the Treasury Board of Canada Secretariat guidance;
- It is based on the department’s strategic outcome(s) and Program Activity Architecture that were approved by the Treasury Board;
- It presents consistent, comprehensive, balanced and reliable results;
- It provides a basis of accountability for the results achieved with the resources and authorities entrusted to it; and
- It reports finances based on approved planned spending numbers from the Treasury Board of Canada Secretariat.
Sharon A. Watts
President and Chief Executive Officer
Summary Information
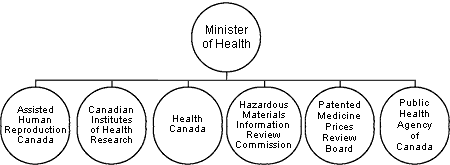
Health Portfolio Overview
The Minister of Health is responsible for maintaining and improving the health of Canadians. This is supported by the Health Portfolio, which comprises Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board, and Assisted Human Reproduction Canada. Each member of the Portfolio prepares its own Report on Plans and Priorities.
The Health Portfolio consists of approximately 12,000 full-time equivalent employees and an annual budget of over $3.8 billion.
Raison d’être
The Hazardous Materials Information Review Commission (HMIRC) provides a mechanism for protecting the trade secrets of those companies that manufacture, supply and/or use hazardous materials and to accurately inform Canadian employees who work with such materials about the intrinsic health and safety hazards
Mandate
The Hazardous Materials Information Review Act mandates the Commission to:
- Register claims for trade secret exemptions and issue registry numbers;
- Adjudicate and issue decisions on the validity of claims for exemption using prescribed regulatory criteria;
- Make decisions on the compliance of Material Safety Data Sheets (MSDSs) and labels to Workplace Hazardous Materials Information System (WHMIS) requirements; and
- Convene independent boards with representatives drawn from labour, suppliers or employers to hear appeals from claimants or affected parties on decisions and orders.
Mission
The Commission’s mission is to:
- Ensure a balance between industry’s right to protect confidential business information and the right of employers and workers to know about the hazardous materials they deal with in the workplace;
- Provide a trade secret mechanism within WHMIS; and
- Resolve complaints and disputes impartially, fairly and promptly through statutory or alternate means.
Vision
HMIRC has defined its vision as:
- Making decisions based on both sound scientific principles and regulations, and taking pride in being a professional quasi-judicial organization seeking creative and progressive approaches to enhancing workplace safety; and
- Resolving complaints and disputes, whether under statutory mandate or not, in a manner that is impartial, fair and prompt.
Values and operating principles
The Commission recognizes that continuous improvement is critical in order to remain relevant and to provide effective and efficient performance and service quality. HMIRC has identified the values and operating principles that foster continuous improvement in its operations.
FAIRNESS–in its ability to provide services and to perform statutory functions.
TIMELINESS–in its ability to provide services within established and reasonable time frames.
ACCESSIBILITY and TRANSPARENCY–in its ability to provide information and services simply and clearly and with policies and procedures that are understandable to everyone.
ACCOUNTABILITY–in its ability to propose legislative approaches only when they meet rigorous cost-benefit analysis and to be accountable for programs and the impact of decisions, while providing services in a manner that is cost-effective for everyone involved.
QUALITY and CONSISTENCY–in its ability to render accurate, relevant, dependable, understandable, predictable and error-free decisions, while ensuring consistent, firm enforcement of the regulations.
COMPETENCY and RESPECT–in its ability to provide services based on a high level of skill, knowledge, scientific and technical competence, and to demonstrate respect and professionalism to everyone who comes into contact with the Commission.
SECURITY and CONFIDENTIALITY–in its ability to store and handle the trade secrets of its claimants.
Context
Labour, industry and government agree on the importance of reducing illnesses and injuries from hazardous materials in Canadian workplaces. WHMIS, a combination of laws, regulations and procedures, was created in 1987 to help achieve this goal.
WHMIS requires suppliers-including manufacturers, importers and distributors-and employers to provide information on the hazards of chemicals produced or used in Canadian workplaces. It requires cautionary labelling for containers of controlled (hazardous) products as designated under federal regulations, and requires that suppliers provide an MSDS for each product.
Each MSDS must include several types of information. For example, it must list all hazardous ingredients in the product, any toxicological properties, the safety precautions workers need to take when using the product, and first-aid treatment in case of exposure. Employers must provide their employees with this MSDS information, as well as with training and education programs.
When labour, industry and government agreed to create WHMIS, they recognized the need to balance the rights of workers and employers to have health and safety information against the rights of chemical suppliers to protect confidential business information.
The Hazardous Materials Information Review Act and its regulations provide the mechanism to create that balance through HMIRC. The Commission is an independent agency with a quasi-judicial role that supports WHMIS responsibilities and interests of the federal, provincial and territorial governments, workers, employers, and the chemical industry.
Role of the Commission
If a supplier or employer wants to withhold information that it believes to be a trade secret, it must file a claim with the Commission for exemption from its WHMIS obligations to disclose this information. The screening officers review these claims against the applicable federal, provincial or territorial regulations, and rule on the claims’ validity. This process involves communication between evaluators, screening officers and claimants to ensure transparency.
As part of this claim review process, the scientific evaluators play a key health and safety role. They review all the information provided on the MSDSs and labels associated with a claim for exemption to make certain that they provide appropriate health and safety information and guidance to comply with WHMIS requirements, based on the Hazardous Products Act, the Canada Labour Code, the Controlled Products Regulations, and provincial and territorial occupational health and safety legislation. This helps ensure that workers are informed of the hazards of exposure to chemicals found in products associated with claims for exemption. When evaluators identify missing or incorrect information, they advise the screening officers who issue formal orders requiring that claimants make the necessary changes and submit the corrected MSDS within 75 calendar days.
The Commission also convenes independent boards to hear appeals from claimants or affected parties challenging decisions and orders.
In addition, HMIRC responds to requests from federal, provincial or territorial government health and safety officials for information about claims for exemption to help these officials administer and enforce their WHMIS obligations.
A model partnership of key stakeholders across all jurisdictions
HMIRC deals with many WHMIS stakeholders:
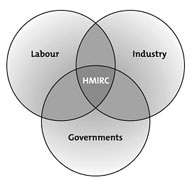
- Labour organizations and workers;
- Suppliers in the chemical industry;
- Employers with workplace WHMIS programs; and
- Federal, provincial and territorial government agencies with WHMIS responsibilities.
As an independent agency, the Commission is a model of industry, labour and government consultation, consensus and cooperation. Its adjudicative efforts must result in a fair balance between the right of workers to know and the right of suppliers and employers to safeguard confidential business information. The Commission makes a tangible contribution to worker health and safety and is a strategic partner to industry and employers. HMIRC’s work also supports the federal, provincial and territorial governments in the delivery of their occupational safety and health regulatory activities, making the Commission one of very few adjudicative bodies that represent multiple levels of government in Canada.
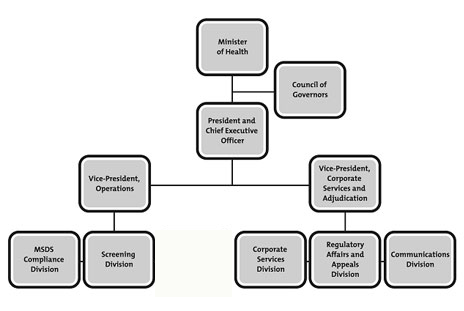
Governance structure
The Commission’s governance structure is one of collaboration. The Council of Governors constitutes the key element of the Commission’s governance structure, acts as an advisory body and provides strategic advice and guidance to the Commission.
The Council of Governors consists of up to18 members representing key stakeholders across all jurisdictions:
- 2 representing workers,
- 1 representing suppliers,
- 1 representing employers,
- 1 representing the federal government, and
- Between 4 and 13 representing the provincial and territorial governments responsible for occupational health and safety.
Most Council members concurrently represent other occupational health and safety organizations, and thus are part of the existing multi-jurisdictional occupational health and safety network.
The Commission’s President and Chief Executive Officer is appointed by the Governor in Council and has the authority to supervise and direct the organization’s day-to-day activities.
The Vice-President of Operations directs the work of the MSDS Compliance and Screening divisions.
The Vice-President of Corporate Services and Adjudication directs the work of the Corporate Services, Regulatory Affairs and Appeals, and Communications divisions.
Organizational Chart:
Voted and Statutory Items displayed in the Main Estimates
| Vote or Statutory Item | Truncated Vote or Statutory Wording | 2008–09 Main Estimates ($ Thousands) |
2007–08 Main Estimates ($ Thousands) |
|---|---|---|---|
| 30 | Program expenditures | 3,097 | 3,024 |
| (S) | Contributions to employee benefit plans | 468 | 482 |
| Total Agency | 3,565 | 3,506 |
The difference between the 2008–09 and the 2007#8211;08 Main Estimates is the collective agreement funding.
Departmental Planned Spending Table and Full-time Equivalents
| ($ thousands) | Forecast Spending 2007–2008 | Planned Spending 2007–2008 | Planned Spending 2009–2010 | Planned Spending 2010–2011 |
|---|---|---|---|---|
| Claims Exemption Process | 3,506 | 3,565 | 3,565 | 3,565 |
| Budgetary Main Estimates (gross) | 3,506 | 3,565 | 3,565 | 3,565 |
| Non-budgetary Main Estimates (gross) | – | – | – | – |
| Less: Respendable revenue | – | – | – | – |
| Total Main Estimates | 3,506 | 3,565 | 3,565 | 3,565 |
| Adjustments | ||||
| Supplementary Estimates | ||||
| Operating budget carry forward | 151 | – | – | – |
| Other | ||||
| Treasury Board Vote 15 | 73 | – | – | – |
| Employee Benefit Plan (EBP) | 15 | – | – | – |
| Total Adjustments | 239 | – | – | – |
| Total Planned Spending | 3,745 | 3,565 | 3,565 | 3,565 |
| Less: Non-respendable revenue | (570) | (570) | (570) | (570) |
| Plus: Cost of services received without charge | 750 | 761 | 761 | 761 |
| Total Departmental Spending | 3,925 | 3,756 | 3,756 | 3,756 |
| Full-time Equivalents | 35 | 35 | 35 | 35 |
The spending trend remains constant to meet the obligations of the Commission’s four priorities over the subsequent three years.
Summary Information
| 2008–2009 | 2009–2010 | 2010–2011 |
|---|---|---|
| 3,565 | 3,565 | 3,565 |
| 2008–2009 | 2009–2010 | 2010–2011 |
|---|---|---|
| 35 | 35 | 35 |
| Name | Type |
|---|---|
| 1. Efficient Client Service Delivery | Ongoing |
| 2. Management Excellence | Ongoing |
| 3. Modernized Legislation | Previously committed |
| 4. International Harmonization | Previously committed |
| Planned Spending ($ thousands) |
|||||
|---|---|---|---|---|---|
| Expected Results | 2008–2009 | 2009–2010 | 2010–2011 | Contributes to the following priority | |
| Strategic Outcome: | Trade secret exemptions are provided in a way that balances the right of industry to protect their confidential business information with the right of workers to receive accurate information concerning the health and safety hazards posed by chemicals in the workplace. | Priorities 1, 2, 3 and 4 | |||
| Claims Exemption Process | Under this activity, the Hazardous Materials Information Review Commisson registers claims for exemption received from a supplier or employer who wishes to withhold confidential business information, decides on the validity of the claim, adjudicates and issues decisions on the compliance of the material safety data sheet (MSDS) or label to which the claim relates, and administers an appeal process to these decisions. | 3,565 | 3,565 | 3,565 | |
Departmental Plans and Priorities
HMIRC’s four priorities for the next three years are:
- Priority 1: Efficient Client Service Delivery
- Priority 2: Management Excellence
- Priority 3: Modernized Legislation
- Priority 4: International Harmonization
Over the next three years, the Commission will focus on these four priorities with particular emphasis on expected improvement to the claims exemption process-the Commission’s key program activity-and the reduction of the backlog of claims at the Commission. Improvements will be achieved through continued efficiencies in the delivery of client services, enhancements in management practices, and legislative renewal with the goal of expediting the availability of complete and accurate MSDSs, submitted to the Commission for review, in Canadian workplaces.
Priority 1: Efficient Client Service Delivery
As an organization with only one program activity, HMIRC’s capacity to process claims as efficiently as possible directly impacts the service that can be provided to clients and stakeholders. For this reason, the Commission is committed to the continuous improvement of claim processing so that clients can market their products without delay, claims can be completed within shorter turn-around times, and employers and workers can have access to accurate MSDSs as quickly as possible.
HMIRC has been experiencing a significant increase in both the number and the complexity of claims in recent years. This, coupled with a chronic shortage of qualified scientific personnel and insufficient resources in supporting areas, has resulted in a claim-processing backlog of approximately two years. This backlog is causing considerable delay in the provision of the corrected health and safety information to Canadian workers and, as a consequence, placing them at significantly higher risk. In order to address this issue, the Commission has developed a plan to prevent further increases to the backlog, eliminate it over a three-year period, and to prevent it from recurring.
In 2007#8211;08, the Commission undertook a comprehensive review of its claims exemption process with the intent to optimise the current method of assessing claims. The objective of the strategic review was to ensure that HMIRC had the most efficient claims assessment system possible, allowing for the communication of decisions to claimants within the shortest time frame possible. The review identified three initiatives that would significantly improve the process: the development of an integrated data management system; the development and application of new tools for evaluators and screening officers; and an aggressive human resources strategy involving recruitment, training, development, and retention components to maintain a stable workforce.
Acting on the results of the review, the Commission will focus on the development of an integrated data management system that will allow quick access by Commission staff to all documents relevant to the assessment of a claim for exemption. Information such as scientific publications, MSDSs, toxicology summaries, Advice Documents (ADs), relevant Acts and Regulations, and guidance and policy documents will be made immediately available electronically-saving valuable time for evaluators.
The Commission also plans to develop and apply a number of new tools to expedite claim processing. For example, an electronic template and guidance manual will standardize AD preparation across the Commission and facilitate the peer-review and subsequent drafting of orders to correct violations on MSDSs. It is expected that this will significantly enhance the current operating procedures and positively impact the production of ADs.
An aggressive human resources strategy will be developed and implemented to address the past challenges of recruiting and retaining HMIRC’s health and safety evaluators that conduct assessments of MSDSs. The evaluators at the Commission are in a scientific group classified as a shortage group in the federal public service, and are difficult to replace and retain. The Commission’s human resources strategy involves actively pursuing recruitment methods to quickly fill vacant or new positions with minimal delay. Some of the methods that will be optimized include selecting candidates from pre-qualified pools within the public service, staffing from the Public Service Commission’s inventory of Scientific and Professional database, recruiting new post-graduates from Canadian universities, and collective staffing in collaboration with other partners within the health portfolio.
A training and development program will also be implemented to train new staff to become fully functional at their group and level within the shortest time possible and to embark on a developmental program in order to be ready for higher-level functions. Included in the training and development of new staff will be the new tools developed to expedite the claim assessment process and a one-on-one coaching system where the new employee is paired with a senior staff member who mentors the individual through the techniques used in claim evaluations. This close one-on-one coaching technique has been piloted within the Commission for a six-month period and has shown a significant reduction in the time required to train new staff.
These three initiatives identified in the strategic review (integrated data management system, new tools for evaluators and screening officers, new human resources strategy) will help maintain a steady workforce and keep claim processing productivity at a level that meets demand in order to avoid further increases to the backlog.
In addition, the Commission is exploring options in order to acquire the resources necessary to develop and implement the three initiatives identified in the strategic review. These resources will also provide additional screening and evaluation resources to increase claim-processing capacity to help eliminate the backlog, and enhanced monitoring and performance measurement methods will be put in place to ensure that a backlog of similar magnitude is prevented from recurring.
Priority 2: Management Excellence
As a small organization with a limited budget, the Commission is highly focused on service delivery. Our ongoing objective is to continue to integrate strategic and business planning with human resource planning. The Commission continues to work collaboratively with its Health Portfolio partners, particularly Health Canada, on portfolio and government management issues.
In a government environment that has a strong commitment to improving service and accountability to the Canadian public by implementing management initiatives such as the Treasury Board Policy Suite Renewal, the Federal Accountability Act, and the Public Service Modernization Act, the Commission continues to be challenged to appropriately address these initiatives while delivering its program activity - Claims Exemption Process. These management initiatives are resource intensive and greatly impact the Commission’s limited resource capacity.
To respond appropriately to these initiatives, while maintaining service delivery, HMIRC will continue along the established path of solidifying strategic partnerships by collaborating and networking horizontally with other departments. This allows the Commission to access interdepartmental resources and expertise through such vehicles as Memoranda of Understanding. As requirements from these initiatives progress, HMIRC will refine its performance management systems while monitoring performance.
The Commission will continue to improve program management by enhancing the tools and practices to provide timely monitoring and reporting. Performance management systems focus on the development of sound and practical performance agreements for management cadres and ensure alignment with the business and human resources goals and priorities. HMIRC will continue its work in strengthening our expenditure management system and use it as its management and reporting tool, following Treasury Board Secretariat Management, Resources, and Results Structure (MRRS)’s initiative. The focus will be to enhance effectiveness and efficiency. The Commission’s performance measurement framework is an essential tool in the monitoring of results and improvements to the quality and completeness of incoming claims and, therefore, processing efficiencies.
Key to delivering on its mandate is HMIRC’s ability to build and maintain workforce capacity. Retention efforts of the Commission are important not only in the area of scientific expertise, but also in management support, which is the foundation of the organization in ensuring efficient systems, processes and procedures. In line with this goal, HMIRC will ensure that the talent required is recruited, and that it has the systems in place to develop human resources so that they have the skills required for the future and that leadership is fostered at all levels. The Commission will focus on development as well as recruitment to meet future requirements. This will allow the Commission to better serve clients and lead to results for Canadians.
Priority 3: Modernized Legislation
On March 29, 2007, Bill S-2 amending the Hazardous Materials Information Review Act received Royal Assent and became Chapter 7 of the Statutes of Canada 2007. The effect of these amendments will be to reduce the time required to review claims for exemption from disclosure of confidential information, speed up the correction of the information workers need in order to handle hazardous materials safely, and expedite and improve the appeal process.
Throughout the renewal process, the Commission has been working closely with stakeholders through its tripartite governing body, the Council of Governors. Consequential to the changes to the Commission’s statute, amendments to the Hazardous Materials Information Review Regulations and Hazardous Materials Information Review Act Appeal Board Procedures Regulations are anticipated to be completed by Fall 2008, at which point both legislative and regulatory changes will take effect. The Commission is already in the process of adjusting its operational processes to the revised procedures outlined in the amended legislation and regulations in order to ensure a seamless transition. The implementation of these amendments will complete the legislative commitments made to stakeholders.
The amendments are closely linked to HMIRC’s efforts to provide effective and efficient services to its stakeholders through its participation in the government-wide Paperwork Burden Reduction Initiative (PBRI). The PBRI involves measuring the costs and impact of regulatory compliance on businesses and pursuing opportunities to reduce, rationalize and simplify regulatory requirements across federal departments and agencies. HMIRC is working with its Health Portfolio partners to measure the impact of regulatory compliance on businesses and make measurable reductions in paperwork burden.
HMIRC is also involved in two other projects concerning changes to legislation in order to better serve Canadians. Firstly, the Commission is engaged in amendments to the Hazardous Products Act that will involve separating the legislation governing consumer products and from the legislation outlining WHMIS. The result will be two distinct and improved pieces of legislation. This will be an excellent opportunity to integrate enhanced safety standards that have been under development in recent years.
Secondly, HMIRC is actively involved in the development of a Canadian implementation strategy for the Globally Harmonized System for the Classification and Labelling of Chemicals (GHS). The GHS is a United Nations initiative to harmonize approaches to classification and labelling of chemicals worldwide. The international community has agreed that countries should make the necessary changes to their own legislation and processes to make the system operational by 2008.
Priority 4: International Harmonization
As mentioned in Priority 3, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is a United Nations initiative to enhance the protection of human health and the environment by providing an internationally comprehensible system for hazard communication. Canada continues to undertake measures for GHS implementation in order to work towards the goal of achieving harmonization in 2008.
The Commission plays a key role in helping to foster progress towards GHS implementation in Canada. As a member of several committees, national working groups, and technical sub-committees, the Commission provides guidance and advice on classification and hazard communication related to workplace chemicals. It occupies a unique place as Canada’s only agency that manages the registration of claims by industry for protection of confidential business information on hazardous workplace chemicals. The Commission is working in collaboration with Health Canada, Transport Canada, and other government departments and agencies on this initiative.
While many international issues are being addressed, Canada’s trade secret exemption process for workplace hazardous materials meets GHS requirements and therefore no changes to it are necessary. However, classification and hazard communication are key elements of HMIRC’s reviews and therefore, while the GHS may not have an impact on the trade secret exemption process, there may be important operational impacts on the MSDS review process and related standards, and may require legislative amendments to the Commission’s enabling statute.
Although the nature and scope of its potential impacts are not clearly defined at this point, GHS implementation will likely result in significant work and training on the part of the Commission in an effort to accommodate the new requirements for hazard communication in MSDSs and labels.
The Commission’s focus over the next three years will be to ensure that it can be responsive to the impending changes and to maintain an international presence. It will continue to work with other Canadian governmental agencies to communicate the benefits of Canada’s WHMIS model to HMIRC’s international partners, particularly the advantages of Canada’s trade secret protection mechanism. In order to maintain an understanding of hazard communication at the international level, the Commission will develop a comparative framework depicting national trade secret protection mechanisms in GHS participating countries in relation to the Canadian model.
