Common menu bar links
Breadcrumb Trail
ARCHIVED - RPP 2007-2008
Patented Medicine Prices Review Board
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Section III - Supplementary Information
Health Portfolio
The Health Portfolio contributes to specific dimensions of improving the health of Canadians. It comprises Health Canada and five agencies, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board, and the newly established Assisted Human Reproduction Canada. T he PMPRB is a member of the Health Portfolio that carries out its mandate at arm's-length from the Minister of Health.
The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act as set out in sections 79 to 103. The Minister is responsible for tabling reports in Parliament on the activities of the PMPRB including, the Report on Plans and Priorities, the PMPRB's Annual Report and the Departmental Performance Report. The Minister also recommends to the Governor-in-Council the appointment of Board Members and changes to the Patented Medicines Regulations, 1994. He/she may also request the Board to conduct inquiries under Section 90 of the Patent Act.
Organizational Information
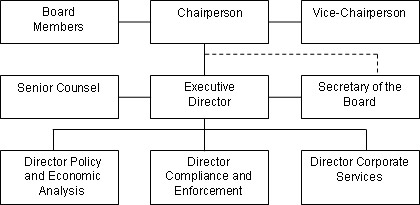
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and Vice-Chairperson. In June 2006, the Vice-Chairperson, Dr. Benoit, was appointed as Chairperson and Mary Catherine Lindberg was appointed Member and Vice-Chairperson for a 5 year term. At present one Board position remains vacant.
The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. The Executive Director manages the work of the staff. Senior staff consists of the Executive Director, the Director of Compliance and Enforcement, the Director of Policy and Economic Analysis, the Director of Corporate Services, the Secretary of the Board and Senior Counsel.
The Compliance and Enforcement Branch is responsible for the review of patented medicine prices and implementing the Compliance and Enforcement Policy. The Policy and Economic Analysis Branch is largely responsible for conducting analyses and preparing reports on price trends and other economic studies. The Secretariat, Corporate Services Branch and Senior Counsel provide Board communications, administrative and legal support, respectively. The Secretariat also provides support to the Board Members and manages the hearing process.
PMPRB links to the Government of Canada Outcomes
| 2007-2008 | ||||||
| ($ thousands) Program Activity |
Budgetary | Total Main Estimates |
Total Planned Spending | |||
| Operating | Gross | Respendable Revenue | Net | |||
| Patented Medicine Prices Review | 11,475.0 | 11,475.0 | - | 11,475.0 | 11,475.0 | 11,475.0 |
| Total | 11,475.0 | 11,475.0 | - | 11,475.0 | 11,475.0 | 11,475.0 |
The Program Activity contributes to the achievement of the Government of Canada's “Healthy Canadians” outcome area.
Table 1: PMPRB Planned Spending and Full Time Equivalents
| ($ thousands) | Forecast Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
Planned Spending 2009-2010 |
| Patented Medicine Prices Review | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Budgetary Main Estimates (gross) | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Less: Respendable revenue | - | - | ||
| Total Main Estimates | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Adjustments: | ||||
| Procurement Savings | ||||
| Patented Medicine Prices Review | ||||
| Supplementary Estimates | ||||
| Program Integrity | 4,915.0 | - | - | - |
| Budget Carry-forward | 177.0 | |||
| Budget Announcement | ||||
| Other | ||||
| Treasury Board Vote 15 | 85.9 | |||
| Employee Benefit Plan (EBP) | 17.1 | |||
| Total Adjustments | 5,195.0 | - | - | - |
| Total Planned Spending | 11,707.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Total Planned Spending | 11,707.0 | 11,475.0 | 4,989.0 | 4,948.0 |
| Less: Non-Respendable revenue | (210.0)9 | - | - | - |
| Plus: Cost of services received without charge | 854.5 | 933.2 | 752.2 | 740.3 |
| Net cost of Program | 12,315.5 | 12,408.0 | 5,741.2 | 5,729.3 |
| Full Time Equivalents | 62.8 | 62.0 | 39.0 | 39.0 |
Table 2: Voted and Statutory Items
| 2007-2008 ($ thousands) |
|||
| Vote or Statutory Item | Truncated Vote or Statutory Wording | 2007-2008 Main Estimates |
2006-2007 Main Estimates |
| 30 | Operating expenditures | 10,584.0 | 5,800.0 |
| (S) | Contributions to employee benefit plans | 891.0 | 712.0 |
| Total Agency | 11,475.0 | 6,512.0 | |
Table 3: Services Received Without Charge
| ($ thousands) | 2007-2008 |
| Accommodation provided by Public Works and Government Services Canada (PWGSC) | 592.1 |
| Contributions covering employers' share of employees' insurance premiums and expenditures paid by Treasury Board of Canada Secretariat (excluding revolving funds) | 337.1 |
| Salary and associated expenditures for legal services provided by Department of Justice Canada | 4.0 |
| Total 2007-2008 Services received without charge | 933.2 |
Table 4: Sources of Non-Respendable Revenue
| ($ thousands ) | Forecast Revenue 2006-2007 |
Planned Revenue 2007-2008 |
Planned Revenue 2008-2009 |
Planned Revenue 2009-2010 |
|
Patented Medicine Prices Review |
||||
| Voluntary Compliance Undertakings | 210.0 10 | - | - | - |
| Total Non-respendable Revenue | 210.0 | - | - | - |
1 The reduction in the estimated planned spending and FTEs for 2008-2009 and beyond is due to the expiration of the Memorandum of Understanding between the PMPRB and Health Canada to fund the PMPRB's work under the NPDUIS and a reduction in funding the PMPRB received to enhance its price review process through Health Canada's Therapeutic Access Strategy. It is expected that new funding arrangements will have been negotiated prior to the expiration of the current MOU. In 2006, through Supplementary Estimates, the PMPRB received additional funding over 2 years to address workload and cost pressures resulting from a number of hearings before the Board. This funding is for 2006-2007 and 2007-2008.
2 The planned spending total includes an amount, prorated by number of FTEs, for centralized Management and Administration costs.
3 Funding for the NPDUIS and reporting on non-patented prescription drugs is reduced slightly because the rate used to calculate the employee benefit plan (EBP) was reduced to 18.5% from the 20% used to calculate the original transfer.
4 Additional information on the Guidelines c an be found in Chapter 1 of the Compendium of Guidelines, Policies and Procedures which is available on the PMPRB website: www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
5 Additional information on the criteria for commencing an investigation can be found in Schedule 5 of the Compendium of Guidelines, Policies and Procedures which is available on the PMPRB website:
www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
6 The reduction in the estimated planned spending and FTEs for 2008-09 and beyond is due to the expiration of the Memorandum of Understanding between the PMPRB and Health Canada to fund the PMPRB's work under the NPDUIS and a reduction in funding the PMPRB received to enhance its price review process through Health Canada's Therapeutic Access Strategy. It is expected that new funding arrangements will have been negotiated prior to the expiration of the current MOU. In 2006, through Supplementary Estimates, the PMPRB received additional funding over 2 years to address workload and cost pressures resulting from a number of hearings before the Board. This funding is for 2006-2007 and 2007-2008.
7 The PMPRB has no authority over the prices of non-patented drugs, including generic drugs sold under compulsory licenses, nor does it have jurisdiction over prices charged by wholesalers or retailers, or over pharmacists' professional fees. Also, matters such as distribution and prescribing are outside the purview of the PMPRB.
8 See the PMPRB's A description of the Laspeyres Methodology used to construct the Patented Medicine Price Index (PMPI), March 1997, revised June 2000, for a detailed explanation of the PMPI. The PMPI measures the overall change in the prices of existing patented drug products, and is constructed by taking a sales-weighted average of rates of price change at the level of individual products. It is not designed to measure the effects of changes in the quantities of drugs consumed or substitution among drugs (for example, the use of newer drugs in place of older, and possibly less costly drugs) on sales. As of the 1999 Annual Report, the PMPI encompasses prices of patented drugs for human use only.
9 The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made be patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province or territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
10 The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made be patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province/territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
