ARCHIVED - RPP 2006-2007
Assisted Human Reproduction Agency of Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
The Honourable Tony Clement
Minister of Health
Minister's Message
Management Representation Statement
Program Activity Architecture (PAA) Crosswalk
Summary Information
Departmental Plans and Priorities
Section II - Analysis of Program Activities by Strategic Outcome
Analysis by Program Activity
Strategic Outcome:
Licencing and Enforcement of a Regulatory Framework for AHR technologies
Health Information and Knowledge Management for Assisted Human Reproduction Technologies
Performance Measurement
Section III - Supplementary Information
Organizational Information
Table 1: Departmental Planned Spending and Full Time Equivalents
Table 2: Program Activities
Table 3: Voted and Statutory Items listed in Main Estimates
Table 4: Internal Audits and Evaluations
Section I - Overview
Minister's Message
 Canadians expect that the benefits of assisted human
reproductive technologies and research must be balanced by protecting the health, safety and dignity of those individuals who access these technologies.
Canadians expect that the benefits of assisted human
reproductive technologies and research must be balanced by protecting the health, safety and dignity of those individuals who access these technologies.
In 2004, through the promulgation of the Assisted Human Reproduction Act, the Government of Canada demonstrated its commitment to this principle and the need to protect human individuality, diversity and the integrity of the human genome.
The legislation brought into force prohibitions against unacceptable practices such as human cloning, together with other measures protecting the health and safety of individuals using AHR to help them build their families. The Act also included provisions to ensure that research involving the in-vitro human embryo in Canada would take place within a controlled environment, and established the new Assisted Human Reproduction Agency of Canada (AHRAC) to implement these safeguards.
Located in Vancouver, B.C., the Agency will licence AHR controlled activities, enforce compliance with the regulations, monitor trends and developments, collect and safeguard personal health information and advise the Minister of Health on these matters.
On June 22, 2005 the Government launched an open and transparent process to recruit the board of directors of the new Agency, including its President and Chairperson. This process is expected to culminate in 2006 with the appointments by Governor-in-Council of the successful candidates to the Agency's board of directors.
The legislation and the establishment of the Agency puts Canada in a leadership position internationally as a regulator in this sector. Nevertheless, much work remains over the next year to complete the regulatory framework and to enable the Agency to begin its operations. Health Canada will continue to press forward with its efforts to introduce these regulations.
This first Report on Plans and Priorities for the Agency outlines the immediate priorities for the Agency, to be refined and confirmed by its board of directors, as they take over the governance of the Agency and begin building its organizational capacity over the coming year.
I am confident that the Agency will move quickly to become an important player in providing a welcome contribution to Canada's health system and the overall health of Canadians.
Tony Clement
Minister of Health
Management Representation Statement
I submit for tabling in Parliament, the 2006-07 Report on Plans and Priorities (RPP) for
the Assisted Human Reproduction
Agency of Canada.
This document has been prepared based on the reporting principles contained in Guide for the Preparation of Part III of the 2006-2007 Estimates: Reports on Plans and Priorities and Departmental Performance Reports:
It adheres to the specific reporting requirements outlined in the TBS guidance;
- It is based on the department's approved Program Activity Architecture structure as reflected in its MRRS;
- It presents consistent, comprehensive, balanced and accurate information;
- It provides a basis of accountability for the results achieved with the resources and authorities entrusted to it; and
- It reports finances based on approved planned spending numbers from the Treasury Board Secretariat in the RPP.
Name: Morris Rosenberg
Title: Deputy Minister
Health Canada
Program Activity Architecture (PAA) Crosswalk
This is a new program for 2006-2007. The Agency was established by Governor-in-Council on January 12, 2006 and the program activities planned for 2006-2007 have no carryover from 2005-2006.
Summary Information
|
Reason for Existence - The Assisted Human Reproduction Agency of Canada (AHRAC) was established under the authority of the Assisted Human Reproduction Act. The legislation aims to protect and promote human health, safety, dignity and human rights in the use of assisted human reproduction (AHR) technologies, prohibits unacceptable activities, such as human cloning and places controls over AHR related research. The Agency will be responsible for the issuance and review of licences, the collection and analysis of health reporting information, and to carry out inspections and enforcement related to activities controlled under the Act. |
Financial Resources ($ thousands)
|
2006-2007 |
2007-2008 |
2008-2009 |
|
$9,681 |
$12,009 |
$12,429 |
Human Resources
|
2006-2007 |
2007-2008 |
2008-2009 |
|
44 FTEs |
44 FTEs |
44 FTEs |
Departmental Priorities
|
|
Type |
Planned Spending ($ thousands) | |||
|---|---|---|---|---|---|
|
2006-2007[1] |
2007-2008[2] |
2008-2009[3] |
|||
|
Strategic Outcome: Protection and promotion of the health and safety of Canadians against the risks associated with assisted human reproduction. |
|||||
|
Priority #1 |
New |
Program Activity #1 - Licencing and Enforcement of a Regulatory Framework for AHR Technologies |
4,320 | 5,289 | 7,796 |
|
Priority #2 |
New |
Program Activity #2 - Health Information and Knowledge Management for AHR Technologies |
5,361 | 6,720 | 4,633 |
Departmental Plans and Priorities
The Assisted Human Reproduction Agency of Canada (AHRAC), established on January 12, 2006, is a federal regulatory organization, which is responsible for administering a regulatory framework and regime to oversee AHR controlled activities, as well as to enforce prohibitions under the Act. Its primary functions will be to administer a licensing framework for controlled activities, to conduct enforcement measures to ensure compliance with prohibitions or terms and conditions of licences, to maintain a national personal health information registry, to provide public information on AHR activities and to promote discussion and advice on AHR issues.
AHRAC is about ensuring the health and safety of donors, patients and children born of AHR technologies. This will be accomplished by implementing a 'state of the art' regulatory framework and regime to oversee AHR procedures and related research in Canada. It will also become a focal point for AHR information to policymakers, practitioners, researchers, patients, children and the public.
AHRAC will be at the centre of an AHR regulatory regime that will include the expertise of other organizations in areas of medicine, allied health professions, accreditation, standards, law, consumer representation and provincial and territorial governments. AHRAC will conduct its decision-making and operations within this milieu on the principles of openness, transparency, accountability, ethics and broad-based representation.
AHRAC is established as a federal regulatory agency, which will maintain the integrity of the legislative intent by working in conjunction with Health Canada and stakeholders to carry out the roles and responsibilities as established by the AHR regulatory framework and regime.
AHRAC's immediate priorities will be to fit up the new agency, and then to build capacity to support the implementation of the AHR regulatory framework. An early activity will also include developing a strategic plan and priorities to begin administering the regulatory framework with licencing, enforcement and public information activities as primary endeavors.
During the fiscal year 2006-07, the Agency will focus on establishing its management and governance structures and staffing up as quickly as possible to begin implementing its systems in preparation to becoming operational. It will train staff, develop the organization's strategic plans, and implement its communications and outreach strategies. It will begin managing compliance and enforcement activities through a Memorandum of Understanding with Health Canada and will work with Health Canada to plan for the transition of the personal health information registry. It will also prepare its systems and processes to operate independently, while keeping abreast of progress on the development of the components of the AHR regulatory framework by Health Canada.
Section II - Analysis of Program Activities by Strategic Outcome
Analysis by Program Activity
Protection and promotion of the health and safety of Canadians against the risks associated with assisted human reproduction.
Program Activity:
Licencing and Enforcement of a Regulatory Framework for AHR technologies.
Program activity description and its expected results
Objective
To ensure compliance with the AHR legislative and regulatory framework.
Description
AHRAC would achieve this objective by the following means:
- issuing licences for controlled activities and for facilities used by qualified persons or organizations;
- assessing applications against licence requirements, including scientific and ethical considerations
- conducting periodic inspections of AHR clinics, service providers or research to ensure compliance; and
- enlisting the participation or support of other recognized organizations in the development of other supporting policy instruments (e.g., standards, guidelines, accreditation models, etc.)
Expected Result
The preliminary key results for this program activity include:
- an effective and efficient licencing and inspection regime;
- compliance by medical practitioners and researchers in respect of prohibitions and controlled activities; and
- improved safety and success of the controlled activities undertaken
Financial Resources: ($ thousands)
|
2006-2007 |
2007-2008 |
2008-2009 |
| 4,320 |
$5,289 |
$7,796 |
Human Resources:
|
2006-2007 |
2007-2008 |
2008-2009 |
|
22 FTEs |
18 FTEs |
17 FTEs |
Strategic Outcome:
Protection and promotion of the health and safety of Canadians against the risks associated with assisted human reproduction.
Program Activity:
Health Information and Knowledge Management for Assisted Human Reproduction Technologies.
Program activity description and its expected results
Objective
To become a centre of expertise and focal point of AHR information for policymakers, practitioners, patients, children born of AHR procedures, researchers and the Canadian public.
Description
AHRAC would achieve this objective by the following means:
- maintaining a personal health information registry (PHIR) to consolidate health reporting information concerning donors, patients and children born of AHR procedures to allow for a look-back and trace-back mechanism;
- providing ongoing reports of AHR controlled activities, including success rates by AHR clinics and results of research, to enable prospective AHR users to make informed decisions; and
- providing public information on AHR matters or issues via a public website or in other forms such as brochures.
Key Results
The preliminary key results for this program activity include:
- a confidential and secure PHIR;
- improved information for decision-making by practitioners and prospective users of AHR procedures;
- improved access by children born of AHR procedures to information on their genetic history; and
- increased awareness of the Canadian public of AHR issues or problems.
Financial Resources: ($ thousands)
|
2006-2007 |
2007-2008 |
2008-2009 |
| 5,361 |
$6,720 |
$4,633 |
Human Resources:
|
2006-2007 |
2007-2008 |
2008-2009 |
|
22 FTEs |
26 FTEs |
27 FTEs |
Performance Measurement
AHRAC will be responsible for a corporate strategy to ensure timely and accurate information on its results derived from the use of public funds. A Performance Measurement Framework will be fully developed by AHRAC upon completion of the AHR regulatory framework and regime. This Framework would identify and elaborate upon:
- key priorities, results or objectives;
- detailed indicators that underlie the broad indicators of key results and capture short, medium and long-term performance;
- responsibilities for achieving results and for performance measurement;
- time-lines for performance reporting; and
- ongoing means for auditing the quality of performance.
To ensure that opportunities for improvement of effectiveness are identified on a regular basis, the Framework will include a timetable for in-depth evaluation of all program mechanisms and activities.
AHRAC will work closely with federal departments, provincial governments and health agencies, medical and allied health professionals, legal and consumer representatives as well as similar organizations in other countries in developing its Framework. This will ensure the optimal use of existing data sources for collection and sharing of information.
AHRAC will establish interim plans, priorities and goals as it proceeds to implement the AHR regulatory framework in collaboration with Health Canada and stakeholders in the AHR sector. Upon completion of the AHR regulatory framework and regime, AHRAC will be able to determine its key results and set appropriate performance measures for the Agency and other participants in the AHR regime.
AHRAC's strategy for measuring performance will require a strong capacity to collect, analyse and share information with other organizations and across several jurisdictions. This will require decisions regarding appropriate investments in information technology and the maintenance of strong linkages with other players in this field.
Section III - Supplementary Information
Organizational Information
Assisted Human Reproduction Agency of Canada
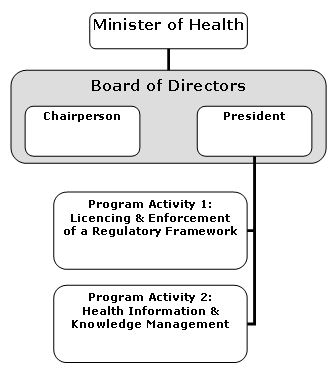
Organizational information as of October 2005
Table 1: Departmental Planned Spending and Full Time Equivalents
($ thousands) |
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|---|---|---|---|---|
|
Licencing and Enforcement of a Regulatory Framework for ahr Technologies |
4,320 | 5,289 | 7,796 | |
|
Health Information and Knowledge Management for Assisted Human Reproduction Technologies |
5,361 | 6,720 | 4,633 | |
|
|
||||
|
Budgetary Main Estimates (gross) |
9,681 | 12,009 | 12,429 | |
|
|
||||
|
Non Budgetary activity |
0 | 0 | 0 | |
|
|
||||
|
Non-Budgetary Main Estimates (gross) |
0 | 0 | 0 | |
|
|
||||
|
Less: Respendable revenue |
0 | 0 | 0 | |
|
Total Main Estimates |
9,681 | 12,009 | 12,429 | |
|
Adjustments: |
|
|||
|
Supplementary Estimates: |
|
|||
|
N/A |
0 | 0 | 0 | |
|
N/A |
0 | 0 | 0 | |
|
Total Planned Spending |
9,681 | 12,009 | 12,429 | |
|
|
|
|||
|
|
||||
|
Total Planned Spending |
9,681 | 12,009 | 12,429 | |
|
Less: Non-Respendable revenue |
0 | 0 | 0 | |
|
Plus: Cost of services received without charge |
0 | 0 | 0 | |
|
Net cost of Program |
9,681 | 12,009 | 12,429 | |
| Full Time Equivalents | 44 | 44 | 44 | |
Table 2: Program Activities
| 2006-2007 ($ thousands) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Budgetary | Non-Budgetary | ||||||||||
|
Program Activity |
Operating |
Capital |
Grants |
Gross |
Contributions and Other Transfer Payments |
Respendable Revenue |
Net |
Loans, |
Total |
Adjustments (planned spending not in Main Estimates) |
Total Planned Spending |
|
Licencing and Enforcement of a Regulatory Framework for AHR Technologies |
4,320 | 4,320 | 4,320 | 4,320 | 4,320 | ||||||
|
Health Information and Knowledge Management for AHR Technologies |
5,361 | 5,361 |
|
5,361 | 5,361 | 5,361 | |||||
|
Total |
9,681 |
|
|
9,681 |
|
|
9,681 |
|
9,681 |
|
9,681 |
Table 3: Voted and Statutory Items listed in Main Estimates
| 2006-2007 | |||
|---|---|---|---|
| Vote or Statutory Item | Truncated Vote or Statutory Wording | Current Main Estimates ($ thousands) |
Previous Main Estimates |
|
10 |
Program expenditures |
$9,022 |
|
|
(S) |
Contribution to employee benefit plans |
659 |
|
|
Total Agency |
$9,681 |
|
|
Table 4: Internal Audits and Evaluations
|
Internal Audits or Evaluations |
|
No audits have been set as the Agency is just beginning operations in the 2006-07 fiscal year. The Agency is required to comply with the Treasury Board's Internal Audit Policy and related directives as applicable. The Agency is expected to report on the performance of all its initiatives on a regular basis through Main Estimates, using the Report on Plans and Priorities and the Agency's Performance Report. The preliminary Program Activity Architecture (PAA) has been developed for AHRAC and will be used to build the Management Resources and Results Structure (MRRS) for the Agency, as a vehicle to report performance. As a new organization, AHRAC is expected to initially focus on the effectiveness of the implementation of the Agency. Subsequently, performance indicators are anticipated to be developed in accordance with the coming into force of regulatory instruments under the AHR Act, to gauge and report on progress towards fulfilling the Agency's legislated and regulatory mandate. The President of the Agency, being the chief executive officer of the Agency, is responsible for the effective management, direction and control of the Agency, including an obligation to ensure that resources allocated for purposes of the Agency are managed well, are being used for priorities identified by Ministers and are achieving results for Canadians. It is expected that the comprehensive parliamentary review of the provisions and operation of the Act, mandated under section 70 of the Act to take place three years after the establishment of the Agency, will in fact be the first opportunity to evaluate progress toward the achievement of desired results. Once the Agency has gained more experience with program delivery, it is anticipated that external resources would be engaged on a periodic basis to provide an independent review of the Agency's operations, addressing management issues relating to the implementation of the Agency, risk management and performance data. |
[1] Excludes PWGSC accommodation charges of $451,000 and EBP charges of $35,000
[2] Excludes PWGSC accommodation charges of $451,000.and EBP charges of $35,000
[3] Excludes PWGSC accommodation charges of $451,000 and EBP charges of $35,000