ARCHIVED - RPP 2007-2008
Patented Medicine Prices Review Board
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
The Honourable Tony Clement
Minister of Health and
Minister for the Federal Economic Development Initiative for Northern Ontario
- Chairperson's Message
- Management Representation Statement
- Program Activity Architecture (PAA) Crosswalk
- Summary Information
- PMPRB Plans and Priorities
Section II - Analysis Of Program Activities By Strategic Outcome
Section III - Supplementary Information
- Health Portfolio
- Organizational Information
- PMPRB links to the Government of Canada Outcomes
- Table 1: PMPRB Planned Spending and Full Time Equivalents
- Table 2: Voted and Statutory Items
- Table 3: Services Received Without Charge
- Table 4: Sources of Non-Respendable Revenue
Section I - Overview
Chairperson's Message
I am pleased to present the 2007-2008 Report on Plans and Priorities for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act. Its mandate is two-fold: the regulatory mandate is to protect consumers and contribute to Canadian health care by ensuring that the prices of patented drugs sold in Canada are not excessive; and the reporting mandate is to contribute to informed decisions and policy-making through reports on pharmaceutical trends, and on research and development spending by pharmaceutical patentees.
From its creation, the PMPRB has been able to carry out its mandate with limited recourse to public hearings. This fact was not a sign of any reluctance on the part of the PMPRB to apply the law, but rather a measure of the success of the Board's Excessive Price Guidelines (Guidelines) and its Voluntary Compliance Policy. In 2006, the Board issued six Notices of Hearing into prices charged by patentees for patented medicines, potentially signaling a new trend.
Recent initiatives such as, the Transparent Drug System for Patient Act, 2006, in Ontario, and the federal/provincial/territorial National Pharmaceuticals Strategy, have brought renewed attention to drug prices and cost trends. At the same time, the PMPRB has heard concerns from many of its stakeholders about high introductory drug prices and other issues. To respond to these concerns, the Board initiated a process to review its Guidelines, including consulting with key stakeholders as required by the Patent Act. The intent of the consultations is to ensure that the Guidelines remain relevant and appropriate in the context of the modern pharmaceutical environment, and that the price review process continues to be transparent and predictable.
In the context of the National Pharmaceuticals Strategy, the PMPRB received direction from the federal Minister of Health to monitor and report on non-patented prescription drug prices. The PMPRB began publishing quarterly reports in July 2006.
The PMPRB is also working in collaboration with the Canadian Institute for Health Information (CIHI) and federal/provincial/territorial jurisdictions to produce analyses and reports under the National Prescription Drug Utilization Information System (NPDUIS). Through critical analyses of price, utilization and cost trends, the PMPRB provides Canada's health system with more comprehensive, timely and accurate information on prescription drug trends and cost drivers.
The PMPRB remains committed to fairness and transparency in the fulfilment of its mandate.

Brien G. Benoit, M.D.
Chairperson
Management Representation Statement
I submit, for tabling in Parliament, the 2007-2008 Report on Plans and Priorities (RPP) for the Patented Medicine Prices Review Board.
This document has been prepared based on the reporting principles contained in the Guide for the Preparation of Part III of the 2007-2008 Estimates: Reports on Plans and Priorities and Departmental Performance Reports:
- It adheres to the specific reporting requirements outlined in the Treasury Board Secretariat guidance;
- It is based on the PMPRB's Strategic Outcome and Program Activity Architecture (PAA) that were approved by the Treasury Board Secretariat;
- It presents consistent, comprehensive, balanced and accurate information;
- It provides a basis of accountability for the results achieved with the resources and authorities entrusted to it; and
- It reports finances based on approved planned spending numbers from the Treasury Board Secretariat in the RPP.

Brien G. Benoit, M.D.
Chairperson
Program Activity Architecture (PAA) Crosswalk
| PAA | Old | New - 2007-2008 |
| Strategic Outcome | Price charged by manufacturers for patented medicines sold in Canada are not excessive | Prices charged by patentees for patented medicines sold in Canada are not excessive and Canadians are informed on pricing trends of medicines, as well as the R&D spending of pharmaceutical patentees. |
| Program Description | The Patented Medicine Prices Review Board (PMPRB) is responsible for regulating the prices that patentees charge, the factory-gate price, for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals or pharmacies for human and veterinary use to ensure that they are not excessive. The PMPRB reports annually to Parliament through the Minister of Health on its major activities, analyses of the prices of patented medicines and of the price trends of all drugs, and reports on the research and development expenditures as reported by patent-holding manufacturers. | The Patented Medicine Prices Review Board (PMPRB) is responsible for regulating the prices that patentees charge, the factory-gate price, for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, or pharmacies for human and veterinary use to ensure that they are not excessive. The PMPRB reports annually to Parliament through the Minister of Health on its major activities, analyses of prices of patented medicines and of the price trends of all drugs, and reports on the research and development expenditures as reported by pharmaceutical patentees. |
The PAA Strategic Outcome was changed firstly, to clearly reflect the dual role of the PMPRB: the protection role; and the reporting role. The second reason for the change was to clarify the PMPRB jurisdiction with respect to patentees as defined in Patent Act (Act).
As per the Act:
“patentee”, in respect of an invention pertaining to a medicine, means the person for the time being entitled to the benefit of the patent for that invention and includes, where any other person is entitled to exercise any rights in relation to that patent other than under a licence continued by subsection 11(1) of the Patent Act Amendment Act, 1992, that other person in respect of those rights.
Subsection 11(1) referred to compulsory licensing arrangements that were in force at the time the legislation was passed - December 1992.
Summary Information
|
Reason for Existence - The PMPRB has a dual role: Regulatory - To protect consumers and contribute to Canadian health care by ensuring that prices charged by patentees for patented medicines are not excessive; Reporting - To contribute to informed decisions and policy making by reporting on pharmaceutical trends and on the R&D spending by pharmaceutical patentees. |
| 2007-2008 | 2008-2009 | 2009-2010 |
| 11,475.0 | 4,989.01 | 4,989.0 |
| 2007-2008 | 2008-2009 | 2009-2010 |
| 62.0 | 39.01 | 39.0 |
| PMPRB Priorities | Type |
| 1. Compliance and enforcement | Ongoing |
|
2. Report on pharmaceutical trends
|
Ongoing |
Program Activities by Strategic Outcome
The PMPRB has one strategic outcome and one program activity. Its objective is to protect consumer interests and contribute to Canadian health care by ensuring that the prices charged by patentees for patented medicines are not excessive, and by reporting on trends in pharmaceutical prices and R&D spending by patentees.
| Planned Spending ($ thousands) |
|||||
| Expected Results | 2007-2008 | 2008-2009 | 2009-2010 | Contributes to the following priority | |
|
Strategic Outcome: |
Prices charged by patentees for patented medicines sold in Canada are not excessive and Canadians are informed on pricing trends of medicines, as well as the R&D spending of pharmaceutical patentees. | ||||
| Patented Medicine Prices Review |
All patentees' prices for new and existing patented medicines sold in Canada are reviewed in a timely manner and in accordance with the PMPRB's Excessive Price Guidelines. |
8,589.52 | 3,435.32 | 3,435.32 | Priority No. 1 |
| Canadian consumers and other stakeholders have complete and accurate information on trends in patentees' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures. | 989.12 | 997.22 | 997.22 | Priority No. 2(a) | |
| Federal/provincial/territorial (F/P/T) drug plans and Canada's health system have more accurate information on prescription drug trends and cost drivers | 1,339.93 | - | - | Priority No. 2(b) | |
| F/P/T Governments and other stakeholders have critical analyses on non-patented prescription drug price trends. | 556.53 | 556.53 | 556.53 | Priority No. 2(c) | |
PMPRB Plans and Priorities
Priority # 1 Compliance and enforcement
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body created by Parliament as a result of revisions to the Patent Act in 1987 (Bill C-22) which increased patent term protection for pharmaceuticals. The PMPRB represents the strategic component of the federal government's policy to protect consumers and contribute to affordable health care in view of other measures designed to protect intellectual property.
The PMPRB is responsible for ensuring that the prices that patentees charge, the “factory-gate” price, for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, pharmacies, or others, for human and veterinary use, are not excessive.
Board Staff reviews the prices of patented medicines sold in Canada to ensure that they are within the Board's Excessive Price Guidelines (Guidelines).4 When it finds that the price of a patented drug product appears to exceed the Guidelines, and the circumstances meet the criteria for commencing an investigation, Board Staff will conduct an investigation to determine the facts.5 An investigation could result in:
- its closure, where it is concluded that the price was within the Guidelines; or
- a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues it received during the period the price was excessive; or
- a recommendation to the Chairperson that it is in the public interest to hold a public hearing to determine if the price is excessive and, if so, for the Board to make a remedial order.
In March 2005, the Board initiated a dialogue with stakeholders on price increases for patented medicines. In response, stakeholders indicated there were pressing issues related to introductory prices for new patented medicines. Further analysis of and consultation on a range of issues is being undertaken by the Board.
In 2006, with the release of its Discussion Guide for the Consultations on the Board's Excessive Price Guidelines, the Board invited stakeholders to focus their comments on the following key issues: the categorization of new drugs; introductory price tests for new drugs; and, how the Board should address the issue of the “any market” clause of the Patent Act in the price review process. Over 40 stakeholders commented on the questions in the Guide. This was followed by stakeholder meetings held in five cities across Canada during November 2006 to hear further views on selected issues flowing from and in addition to the feedback on the Discussion Guide.Board Staff is currently reviewing the information obtained through written submissions and the consultation meetings. An analytical and stakeholder engagement work plan has been developed encompassing a comprehensive range of issues related to the current Guidelines and price review process. The intent is to ensure that the Guidelines remain relevant and appropriate in the context of the modern pharmaceutical environment and that the price review process continues to be transparent and predictable.
Work also continued on the development of proposed amendments to the Patented Medicines Regulation, 1994 (Regulations) which prescribe what information will be filed to the Board as well as the deadlines for doing so. The original proposed amendments to the Regulations were published in the Canada Gazette, Part I on December 31, 2005. Since then, stakeholder comments were analyzed and further revisions were made. Following final publication in the Canada Gazette, Part II, the Regulations will come into force on the day they are registered. At that time, Board Staff will advise patentees of all changes to the filing requirements and will provide revised forms to ensure appropriate implementation of all amendments to the Regulations.
Since January 2006, the Board has issued six Notices of Hearing. These hearings must be conducted in a fair and expeditious manner.
In fiscal year 2006-07, through the Supplementary Estimates, the PMPRB received additional financial resources to conduct public hearings and modernize the Guidelines. This funding, which continues in 2007-2008, is reflected in the Financial Tables in Section III of this report.
Priority # 2 Report on pharmaceutical trends
The PMPRB reports annually to Parliament through the Minister of Health. The PMPRB's Annual Report, which covers the calendar year, includes: a review of its major activities; analyses of prices of patented medicines and price trends of all drugs; and R&D expenditures as reported by patentees. The PMPRB also reports through its quarterly NEWSletter and various studies on both its activities and pharmaceutical trends.
In addition, pursuant to an agreement by the Federal/Provincial/Territorial (F/P/T) Ministers of Health, and at the request of the federal Minister of Health, the PMPRB conducts research under the National Prescription Drug Utilization Information System (NPDUIS). The purpose of the PMPRB's role in the NPDUIS, a partnership initiative with the Canadian Institute for Health Information (CIHI), is to provide critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive and accurate information on how prescription drugs are being used and on sources of cost increases.
In 2006-07, the PMPRB, through its work under the NPDUIS, released the Pharmaceutical Trends Overview Report – Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, and First Nations and Inuit Health Branch of Health Canada, 1997-1998 to 2003-2004 . PMPRB Staff is currently finalizing Guidelines for Budget Impact Analysis and a report on the new drug pipeline.
In October 2005, the Federal Minister of Health, on behalf of himself and his P/T colleagues, directed the PMPRB to monitor and report on the prices of non-patented prescription drugs in Canada. The PMPRB released the first two quarterly reports in June 2006 and October 2006, respectively. In 2007-2008, the PMPRB will continue to produce quarterly reports on: Canadian and foreign non-patented prescription drug price trends; trends in the Canadian market structure for non-patented prescription drugs; trends in market entry of generic prescription drugs, when brand-name products go off-patent; and special studies focussing on topics of interest to F/P/T drug plans.
Section II - Analysis of Program Activities By Strategic Outcome
Analysis by Program Activity
The PMPRB has one strategic outcome and one program activity. Its objective is to protect consumer interests and contribute to Canadian health care by ensuring that the prices charged by patentees of patented medicines are not excessive and by reporting on trends in pharmaceutical prices and R&D spending by patentees.
Strategic Outcome
Prices charged by patentees for patented medicines sold in Canada are not excessive and Canadians are informed on pricing trends of medicines, as well as the R&D spending of pharmaceutical patentees.
Program Activity
Patented Medicine Prices Review
| 2007-2008 | 2008-2009 | 2009-2010 |
| 11,475.0 | 4,989.06 | 4,989.0 |
| 2007-2008 | 2008-2009 | 2009-2010 |
| 62.0 | 39.06 | 39.0 |
The PMPRB is responsible for ensuring that the prices that patentees charge, the “factory-gate” price, for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, pharmacies, and others for human and veterinary use, are not excessive.7
The PMPRB relies on voluntary compliance wherever possible since it is more effective, less time consuming, and less costly to all parties. Voluntary compliance by patentees is facilitated by published Guidelines which are intended to assist companies in setting prices that are not excessive by providing transparent and predictable information on how the price review will be carried out.
The PMPRB reviews the pricing information for all patented medicines sold in Canada on an ongoing basis to ensure that the prices charged by patentees comply with the Excessive Price Guidelines established by the Board. The Guidelines are published in the PMPRB's Compendium of Guidelines, Policies and Procedures (Compendium) and are available on the website: www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
The Guidelines provide a means of operationalizing the price determination factors in section 85 of the Patent Act and have been developed and modified in consultation with stakeholders, including consumer groups, provincial and territorial Ministers of Health, the pharmaceutical industry and others. Section 85 of the Act states:
85. (1) In determining under section 83 whether a medicine is being or has been sold at an excessive price in any market in Canada, the Board shall take into consideration the following factors, to the extent that information on the factors is available to the Board:
- the price at which the medicine has been sold in the relevant market;
- the prices at which other medicines in the same therapeutic class have been sold in the relevant market;
- the prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada;
- changes in the Consumer Price Index (CPI); and
- such other factors as may be specified in any regulations made for the purposes of this subsection.
85. (2) Where, after taking into consideration the factors referred to in subsection (1), the Board is unable to determine whether the medicine is being sold in any market in Canada at an excessive price, the Board may take into consideration the following factors:
- the cost of making and marketing the medicines; and
- such other factors as may be specified in any regulations made for the purposes of this subsection or as are, in the opinion of the Board, relevant in the circumstances.
The Guidelines set out how these factors will be assessed and, include a scientific review to determine to which of the three categories the new medicine belongs and a price review using the price tests established for the medicine category.
The first priority and expected result of the price review program activity is that all patentees' prices for new and existing patented medicines sold in Canada are reviewed in a timely manner and in accordance with the Board's Excessive Price Guidelines
The program activity supports the Government of Canada's Social Affairs outcome of Healthy Canadians by ensuring that Canadians have access to patented pharmaceutical products at prices that are not excessive.
The indicators that show the PMPRB is achieving its expected results and thus its strategic outcome are:
- prices of patented medicines sold in Canada are within the Guidelines;
- compliance and enforcement measures are used to reduce prices that are found to be excessive;
- trends show that price increases for existing patented medicines are not higher than changes in the CPI; and
- trends show that Canadian prices of patented drugs are, on average, below the median international prices in the foreign countries listed in the Patented Medicines Regulations, 1994 (Regulations).
The second priority is that Canadians are informed about pharmaceutical trends. Within this priority the PMPRB has three main responsibilities.
The first responsibility is to report regularly on PMPRB's major activities, on analyses of pharmaceutical prices and price trends, and on research and development expenditures as reported by pharmaceutical patentees.
Patentees are required, under the Regulations, to report their total sales of drugs in Canada, both patented and non-patented, to the PMPRB. Patentees are also required to submit detailed price and volume information, by province/territory and class of customer, on their sales of patented drugs.
The PMPRB maintains the Patented Medicine Price Index (PMPI) to monitor trends in prices of patented drug products sold in Canada based on the average transaction price across Canada.8 It is updated annually using price and sales information reported by patentees.
In accordance with the Patent Act, and the Regulations, patentees must also report all publicly available ex-factory prices of patented drugs in seven foreign countries: France, Germany, Italy, Sweden, Switzerland, the U.K. and the U.S. These data are used to compare patented drug prices in Canada with the median of the foreign prices.
The expected result of this portion of priority two is that Canadian consumers and other stakeholders have complete and accurate information on trends in patentees' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures. This information contributes to informed decisions and policy-making.
The indicator that shows that the PMPRB is achieving this expected result is a comprehensive annual report to Parliament on the aforementioned subjects.
The second major responsibility under priority two pertains to the Minister's direction to the PMPRB under Section 90 of the Act to play a role in the National Prescription Drug Utilization Information System (NPDUIS). This initiative involves preparing critical analyses of prescription drugs so that Canada's health system has more comprehensive and accurate information on how prescription drugs are being used and on sources of cost increases. The Canadian Institute for Health Information (CIHI) and the PMPRB are partners in the NPDUIS. A steering committee, comprised of representatives of F/P/T public drug plans (excluding Quebec) and Health Canada, advises CIHI and the PMPRB on the development of NPDUIS and identifies priorities for analytical studies.
The expected result of this aspect of priority two is that F/P/T drug plans and Canada's health system will have more accurate information on prescription drug price trends, and cost drivers. This information will contribute to informed decisions and policy making about affordable access to quality health care particularly for public drug plans.
The indicator that shows that the PMPRB is achieving this expected result is the number of studies completed under the NPDUIS and how well they are received by F/P/T drug plans.
The third and final responsibility under priority two involves reporting on non-patented prescription drugs. In the fall of 2005, F/P/T Health Ministers agreed that the PMPRB be asked to monitor and report on the prices of non-patented prescription medicines. In October 2005, Health Canada entered into a Memorandum of Understanding with the PMPRB to fund this analysis and in November 2005, the Federal Minister of Health formally directed the PMPRB to undertake this work pursuant to section 90 of the Patent Act.
The PMRPB will produce four quarterly reports each year. These reports will cover a range of topics including sales and price trends in Canada and internationally; a profile of the market structure; entry of generics when brand-name drugs go off-patent; and selected priority topics of interest to F/P/T drug plans.
The expected result of this component of priority two is that F/P/T Governments and other stakeholders will have critical analyses and comprehensive information on non-patented prescription drug prices and market trends.
The indicator that shows the PMPRB is achieving this expected result is completed quarterly reports on non-patented prescription drug prices that are useful in policy analysis for the non-patented drug sector.
Section III - Supplementary Information
Health Portfolio
The Health Portfolio contributes to specific dimensions of improving the health of Canadians. It comprises Health Canada and five agencies, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board, and the newly established Assisted Human Reproduction Canada. T he PMPRB is a member of the Health Portfolio that carries out its mandate at arm's-length from the Minister of Health.
The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act as set out in sections 79 to 103. The Minister is responsible for tabling reports in Parliament on the activities of the PMPRB including, the Report on Plans and Priorities, the PMPRB's Annual Report and the Departmental Performance Report. The Minister also recommends to the Governor-in-Council the appointment of Board Members and changes to the Patented Medicines Regulations, 1994. He/she may also request the Board to conduct inquiries under Section 90 of the Patent Act.
Organizational Information
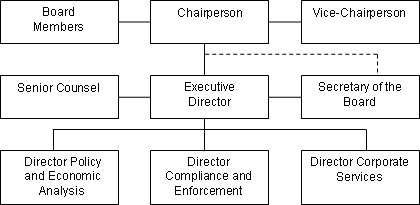
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and Vice-Chairperson. In June 2006, the Vice-Chairperson, Dr. Benoit, was appointed as Chairperson and Mary Catherine Lindberg was appointed Member and Vice-Chairperson for a 5 year term. At present one Board position remains vacant.
The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. The Executive Director manages the work of the staff. Senior staff consists of the Executive Director, the Director of Compliance and Enforcement, the Director of Policy and Economic Analysis, the Director of Corporate Services, the Secretary of the Board and Senior Counsel.
The Compliance and Enforcement Branch is responsible for the review of patented medicine prices and implementing the Compliance and Enforcement Policy. The Policy and Economic Analysis Branch is largely responsible for conducting analyses and preparing reports on price trends and other economic studies. The Secretariat, Corporate Services Branch and Senior Counsel provide Board communications, administrative and legal support, respectively. The Secretariat also provides support to the Board Members and manages the hearing process.
PMPRB links to the Government of Canada Outcomes
| 2007-2008 | ||||||
| ($ thousands) Program Activity |
Budgetary | Total Main Estimates |
Total Planned Spending | |||
| Operating | Gross | Respendable Revenue | Net | |||
| Patented Medicine Prices Review | 11,475.0 | 11,475.0 | - | 11,475.0 | 11,475.0 | 11,475.0 |
| Total | 11,475.0 | 11,475.0 | - | 11,475.0 | 11,475.0 | 11,475.0 |
The Program Activity contributes to the achievement of the Government of Canada's “Healthy Canadians” outcome area.
Table 1: PMPRB Planned Spending and Full Time Equivalents
| ($ thousands) | Forecast Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
Planned Spending 2009-2010 |
| Patented Medicine Prices Review | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Budgetary Main Estimates (gross) | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Less: Respendable revenue | - | - | ||
| Total Main Estimates | 6,512.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Adjustments: | ||||
| Procurement Savings | ||||
| Patented Medicine Prices Review | ||||
| Supplementary Estimates | ||||
| Program Integrity | 4,915.0 | - | - | - |
| Budget Carry-forward | 177.0 | |||
| Budget Announcement | ||||
| Other | ||||
| Treasury Board Vote 15 | 85.9 | |||
| Employee Benefit Plan (EBP) | 17.1 | |||
| Total Adjustments | 5,195.0 | - | - | - |
| Total Planned Spending | 11,707.0 | 11,475.0 | 4,989.0 | 4,989.0 |
| Total Planned Spending | 11,707.0 | 11,475.0 | 4,989.0 | 4,948.0 |
| Less: Non-Respendable revenue | (210.0)9 | - | - | - |
| Plus: Cost of services received without charge | 854.5 | 933.2 | 752.2 | 740.3 |
| Net cost of Program | 12,315.5 | 12,408.0 | 5,741.2 | 5,729.3 |
| Full Time Equivalents | 62.8 | 62.0 | 39.0 | 39.0 |
Table 2: Voted and Statutory Items
| 2007-2008 ($ thousands) |
|||
| Vote or Statutory Item | Truncated Vote or Statutory Wording | 2007-2008 Main Estimates |
2006-2007 Main Estimates |
| 30 | Operating expenditures | 10,584.0 | 5,800.0 |
| (S) | Contributions to employee benefit plans | 891.0 | 712.0 |
| Total Agency | 11,475.0 | 6,512.0 | |
Table 3: Services Received Without Charge
| ($ thousands) | 2007-2008 |
| Accommodation provided by Public Works and Government Services Canada (PWGSC) | 592.1 |
| Contributions covering employers' share of employees' insurance premiums and expenditures paid by Treasury Board of Canada Secretariat (excluding revolving funds) | 337.1 |
| Salary and associated expenditures for legal services provided by Department of Justice Canada | 4.0 |
| Total 2007-2008 Services received without charge | 933.2 |
Table 4: Sources of Non-Respendable Revenue
| ($ thousands ) | Forecast Revenue 2006-2007 |
Planned Revenue 2007-2008 |
Planned Revenue 2008-2009 |
Planned Revenue 2009-2010 |
|
Patented Medicine Prices Review |
||||
| Voluntary Compliance Undertakings | 210.0 10 | - | - | - |
| Total Non-respendable Revenue | 210.0 | - | - | - |
1 The reduction in the estimated planned spending and FTEs for 2008-2009 and beyond is due to the expiration of the Memorandum of Understanding between the PMPRB and Health Canada to fund the PMPRB's work under the NPDUIS and a reduction in funding the PMPRB received to enhance its price review process through Health Canada's Therapeutic Access Strategy. It is expected that new funding arrangements will have been negotiated prior to the expiration of the current MOU. In 2006, through Supplementary Estimates, the PMPRB received additional funding over 2 years to address workload and cost pressures resulting from a number of hearings before the Board. This funding is for 2006-2007 and 2007-2008.
2 The planned spending total includes an amount, prorated by number of FTEs, for centralized Management and Administration costs.
3 Funding for the NPDUIS and reporting on non-patented prescription drugs is reduced slightly because the rate used to calculate the employee benefit plan (EBP) was reduced to 18.5% from the 20% used to calculate the original transfer.
4 Additional information on the Guidelines c an be found in Chapter 1 of the Compendium of Guidelines, Policies and Procedures which is available on the PMPRB website: www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
5 Additional information on the criteria for commencing an investigation can be found in Schedule 5 of the Compendium of Guidelines, Policies and Procedures which is available on the PMPRB website:
www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
6 The reduction in the estimated planned spending and FTEs for 2008-09 and beyond is due to the expiration of the Memorandum of Understanding between the PMPRB and Health Canada to fund the PMPRB's work under the NPDUIS and a reduction in funding the PMPRB received to enhance its price review process through Health Canada's Therapeutic Access Strategy. It is expected that new funding arrangements will have been negotiated prior to the expiration of the current MOU. In 2006, through Supplementary Estimates, the PMPRB received additional funding over 2 years to address workload and cost pressures resulting from a number of hearings before the Board. This funding is for 2006-2007 and 2007-2008.
7 The PMPRB has no authority over the prices of non-patented drugs, including generic drugs sold under compulsory licenses, nor does it have jurisdiction over prices charged by wholesalers or retailers, or over pharmacists' professional fees. Also, matters such as distribution and prescribing are outside the purview of the PMPRB.
8 See the PMPRB's A description of the Laspeyres Methodology used to construct the Patented Medicine Price Index (PMPI), March 1997, revised June 2000, for a detailed explanation of the PMPI. The PMPI measures the overall change in the prices of existing patented drug products, and is constructed by taking a sales-weighted average of rates of price change at the level of individual products. It is not designed to measure the effects of changes in the quantities of drugs consumed or substitution among drugs (for example, the use of newer drugs in place of older, and possibly less costly drugs) on sales. As of the 1999 Annual Report, the PMPI encompasses prices of patented drugs for human use only.
9 The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made be patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province or territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
10 The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made be patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province/territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.