ARCHIVED - RPP 2006-2007
Health Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Tony Clement
Minister of Health, and Minister for the Federal
Economic Development Initiative for Northern Ontario
Section 1: Overview
1.2 Management Representation Statement
1.3 Summary Information
Part A: Departmental Overview and Priorities
Health Canada Planning Framework for 2006-2007 Report on Plans and Priorities
Health Canada's Operational Roles
Our Mission and Objectives
Health Canada and partners helping Canadians to make independent, informed choices
Health Canada's Corporate Priorities
Key Areas of Focus for Planning Period
Operating Principles
Contributing to Government of Canada Strategic Outcomes
Health Canada: Collaboration at Work
Responding to Human Resources Risks and Challenges
Incorporating Sustainable Development Principles into Practice
Performance Measurement Strategy
Key Programs and Services
Intergovernmental
International
Assisted Human Reproduction Implementation Office
Legislation Renewal
Women's Health and Gender Analysis
Applied Research, Dissemination and Accountability
Performance Measurement Strategy
Key Programs and Services
Information, education and outreach on health products, food and nutrition
Monitoring safety and therapeutic effectiveness and risk management
Transparency, public accountability and stakeholder relationships
Performance Measurement Strategy
Key Programs and Services
Drug Strategy and Controlled Substances
Safe Environments
Product Safety
Workplace Health & Public Safety
Program Activity Description - Pest Control Product Regulation
Performance Measurement Strategy
Web Links
Performance Measurement Strategy
Key Programs and Services
Children and Youth Programs
Mental Health and Addictions Programs
Chronic Disease and Injury Prevention Programs
Environmental Health and Research Programs
Communicable Disease Control Programs
Primary Health Care Programs
Table 2: Program Activities for 2006-2007
Table 3: Voted and Statutory Items listed in Main Estimates
Table 4: Services Received Without Charge
Table 5: Sources of Respendable and Non-Respendable Revenues
Table 6: Resource Requirements by Branch and by Program Activity
Table 7: Major Regulatory Initiatives
Table 8: Details on Transfer Payments Programs
Table 9: Conditional Grants (Foundations)
Table 10: Horizontal Initiatives
Table 11: Sustainable Development Strategy
Table 12: Internal Audits and Evaluations
Section 1: Overview
1.1 Minister's Message

I am very pleased to present Health Canada's 2006-2007 Report on Plans and Priorities, which illustrates the initiatives the Department will implement over the next three years to address key government priorities in the area of health. I am honoured to be given the opportunity to work on behalf of Canadians towards ensuring an effective, cost-efficient and high quality health system. Canadians collectively contribute to our public health care system, and all governments have the responsibility to ensure that it is readily available to all Canadians across the country.
Since we were elected to govern in January 2006, our government has adopted six operating principles in its approach to managing the Health Portfolio: putting the patient first in disease prevention and early detection initiatives; making strategic and evidencebased investments; ensuring alignment of policies and programs across the Health Portfolio; building relationships with partners based on trust and inclusiveness; improving performance and ensuring value for money; and strengthening accountability to Parliament and the public. We have already demonstrated our commitment to these principles by agreeing to compensate Canadians who contracted hepatitis C from the blood system before January 1, 1986 and after July 1, 1990.
As Minister of Health, I have a wide array of responsibilities and priorities. As Canadians have made access to health care one of their top priorities, our government has made the Patient Wait Times Guarantee one of its top priorities. The federal government will deliver on this priority in concert with provinces, territories, stakeholders and other partners. I am encouraged by the desire expressed among my provincial and territorial colleagues for innovative and creative ideas to reduce the wait times faced too often by Canadians.
For this reason, Health Canada will work with provinces, territories and other stakeholders to:
- establish further evidence-based benchmarks for wait times in the areas of cancer, heart, diagnostic imaging procedures,
joint replacements and sight restoration;
- encourage provincial wait-reduction targets for priority procedures; and
- provide regular reports to Canadians on progress on wait times.
A Patient Wait Times Guarantee will complement these efforts and build on current and future accomplishments by assuring Canadians that they will receive needed care within appropriate time frames.
Directly linked to reducing wait times, we will make strides on preventing illness and improving disease management across Canada. To this end, we will work with the Public Health Agency of Canada as well as the provinces and territories to implement the Canadian Strategy for Cancer Control to improve cancer screening, prevention and coordination through work with major cancer organizations and stakeholders in Canada.We will also focus efforts in the areas of cardiovascular disease, mental illness and mental health, to name but a few.We will also take action on active living and nutrition, starting with the release later this year of the updated Canada's Food Guide.Work will continue to help ensure safer living and working environments and access to and regulation of pharmaceutical products.
With the SARS outbreak in 2003, we have seen how the health threats that arise outside our borders can quickly pose serious threats to the health of Canadians. These threats are of particular concern because they are nearly impossible to predict and have potentially catastrophic consequences. In partnership with the provinces and territories, First Nations organizations, technical experts and other federal and international partners, I am working to ensure the Government of Canada has an Avian Influenza Plan and a Human Pandemic Influenza Plan in place to mitigate the effects on Canadians in the event of a pandemic. Areas of focus will include avian and pandemic planning, enhanced surveillance capacity, updated quarantine and biosecurity legislation, and infectious disease prevention and control.
In addition to these important areas, we will work on an array of issues fundamental to the health of Canadians. We will strengthen our understanding of the linkages between health and environment, a key concern to Canadians. Health Canada, in partnership with First Nations and Inuit groups, will continue to support sustainable health care services for First Nations on reserve and Inuit people. We will also work with health partners and other federal departments to find new and innovative ways to reduce the health disparities between Aboriginal people and other Canadians. Health research will support the causes and prevention of disease, screening, diagnosis, treatment, support systems and palliative care for a wide range of conditions. For this research, our priority populations are children and youth, seniors and First Nations and Inuit people. We will also improve partnerships and dialogues with international organizations and other countries to help strengthen the Canadian health system.
As our government is determined to provide clear accountability and demonstrate tangible results to Canadians, I have instructed Health Canada to focus on result-based management. While the 2006-2007 Report on Plans and Priorities includes, for the first time, performance indicators for our programs and services to help measure and report on our progress and value for money, I am looking forward to next year's Report to demonstrate further advances on results-based reporting. We will review investments in priority areas to ensure our efforts yield real results that translate into improvements to health for Canadians.
We have established a bold and ambitious agenda but it is no less than what Canadians expect and deserve from their federal government. Through these comprehensive initiatives I am confident that Canada's health system will provide better access to the care one needs and make Canadians among the healthiest people in the world.

1.2 Management Representation Statement
I submit for tabling in Parliament, the 2006-2007 Report on Plans and Priorities (RPP) for Health Canada.
This document has been prepared based on the reporting principles contained in Guide for the Preparation of Part III of the 2006-2007 Estimates: Reports on Plans and Priorities and Departmental Performance Reports:
- It adheres to the specific reporting requirements outlined in the TBS guidelines;
- It is based on the Department's approved Program Activity Architecture as reflected in its Management, Resources and Results Structures (MRRS);
- It presents consistent, comprehensive, balanced and reliable information;
- It provides a basis of accountability for the results achieved with the resources and authorities entrusted to it; and
- It reports finances based on approved planned spending numbers from the Treasury Board Secretariat in the RPP.

1.3 Summary Information
Raison d'être: Health Canada was established to help the people of Canada maintain and improve their health. We are committed to improving the lives of all Canadians and making this country's population among the healthiest in the world as measured by longevity, lifestyle and effective use of the public health care system.
| 2006-2007 | 2007-2008 | 2008-2009 |
|---|---|---|
| 3,011.1 | 2,949.1 | 2,950.3 |
| 2006-2007 | 2007-2008 | 2008-2009 |
|---|---|---|
| 8,711 | 8,773 | 8,671 |
| Strategic Outcome #1: Strengthened Knowledge Base to Address Health and Health Care Priorities Program Activity: Health Policy. Planning and Information | ||||
|---|---|---|---|---|
| Corporate Priority | Planned Spending (in millions of dollars) | Expected Results | ||
| 2006-2007 | 2007-2008 | 2008-2009 | ||
| Working with others to strengthen the efficiency and effectiveness of the publiclyfunded health care system (ongoing) | 217.3 | 146.1 | 144.5 |
|
| Contributing to the improvement of the health of Canadians (ongoing) | 20.7 | 20.6 | 20.5 | |
| Reducing the risks to the health of the people of Canada (ongoing) | 31.7 | 33.6 | 32.3 | |
| Strengthening accountability to Parliament and the public (ongoing) | 18.7 | 17.9 | 17.7 | |
| Strategic Outcome #2: Access to Safe and Effective Health Products and Food and Information for Healthy Choices Program Activity: Health Products and Food | ||||
|---|---|---|---|---|
| Corporate Priority | Planned Spending (in millions of dollars) | Expected Results | ||
| 2006-2007 | 2007-2008 | 2008-2009 | ||
| Working with others to strengthen the efficiency and effectiveness of the publiclyfunded health care system (ongoing) | 102.4 | 102.4 | 97.1 |
|
| Contributing to the improvement of the health of Canadians (ongoing) | 7.3 | 7.3 | 6.9 | |
| Reducing the risks to the health of the people of Canada (ongoing) | 94.7 | 94.7 | 89.8 | |
| Strengthening accountability to Parliament and the public (ongoing) | 57.7 | 54.8 | 54.3 | |
| Strategic Outcome #3: Reduced Health and Environmental Risks from Products and Substances, and
Safer Living and Working Environments Program Activity: Healthy Environments and Consumer Safety |
||||
|---|---|---|---|---|
| Corporate Priority | Planned Spending (in millions of dollars) | Expected Results | ||
| 2006-2007 | 2007-2008 | 2008-2009 | ||
| Working with others to strengthen the efficiency and effectiveness of the publiclyfunded health care system (ongoing) | 33.2 | 33.0 | 33.1 |
|
| Contributing to the improvement of the health of Canadians (ongoing) | 112.1 | 111.6 | 117.2 | |
| Reducing the risks to the health of the people of Canada (ongoing) | 92.2 | 91.8 | 86.7 | |
| Strengthening accountability to Parliament and the public (ongoing) | 52.4 | 49.8 | 49.2 | |
| Strategic Outcome #3: Reduced Health and Environmental Risks from Products and Substances, and
Safer Living and Working Environments Program Activity: Pest Control Product Regulation |
||||
|---|---|---|---|---|
| Corporate Priority | Planned Spending (in millions of dollars) | Expected Results | ||
| 2006-2007 | 2007-2008 | 2008-2009 | ||
| Reducing the risks to the health of the people of Canada (ongoing) | 40.2 | 40.2 | 36.3 |
|
| Strengthening accountability to Parliament and the public (ongoing) | 11.4 | 11.0 | 10.8 | |
| Strategic Outcome #4: Better Health Outcomes and Reduction of Health Inequalities Between
First Nations and Inuit and Other Canadians Program Activity: First Nations and Inuit Health |
||||
|---|---|---|---|---|
| Corporate Priority | Planned Spending (in millions of dollars) | Expected Results | ||
| 2006-2007 | 2007-2008 | 2008-2009 | ||
| Working with others to strengthen the efficiency and effectiveness of the publiclyfunded health care system (ongoing) | 49.6 | 50.1 | 50.5 |
|
| Contributing to the improvement of the health of Canadians (ongoing) | 1,901.4 | 1,920.4 | 1,940.0 | |
| Reducing the risks to the health of the people of Canada (ongoing) | 46.9 | 47.4 | 47.9 | |
| Strengthening accountability to Parliament and the public (ongoing) | 121.2 | 116.3 | 115.3 | |
| Note: Figures include amounts for other departmental and regional infrastructure costs supporting program delivery. | ||||
Part A: Departmental Overview and Priorities
About Health Canada
Health Canada develops, implements and enforces regulations, legislation, policies, programs, services and initiatives and works with other federal partners, the provinces and territories to maintain and improve the overall health of Canadians. As administrator of the Canada Health Act, we ensure that the principles of Canada's universal health care are respected, allowing Canadians to be confident in the services they receive from the public health care system. The Minister of Health is also responsible for the direct administration of another 18 statutes including the Food and Drugs Act, the Pest Control Products Act and the Controlled Drugs and Substance Act. 1
We provide policy leadership and portfolio coordination among our partners in the Government of Canada's Health Portfolio, each of which produces its own Report on Plans and Priorities, namely:
- the Public Health Agency of Canada; 2
- the Canadian Institutes of Health Research; 3
- the Hazardous Materials Information Review Commission; 4
- the Patented Medicine Prices Review Board; 5 and
- the new Assisted Human Reproduction Agency of Canada, which came into being January 12, 2006. 6
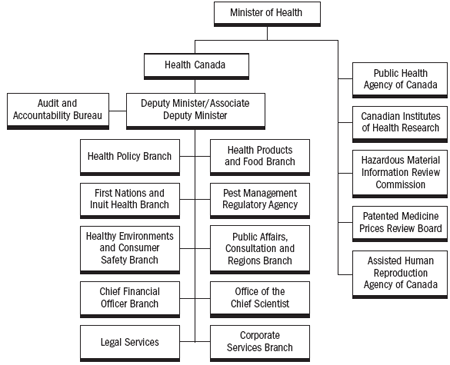
Health Canada also contributes grants and contributions to several health organizations such as Infoway, Canadian Institute for Health Information and Canadian Health Services Research Foundations.
Health Canada Planning Framework for 2006-2007 Report on Plans and Priorities
Health Canada's Operational Roles
Health Canada employees play key roles in the areas of promoting, protecting and improving the health of Canadians, roles that assist other stakeholders working in the area.
Innovators
As a science-based department, Health Canada employees are innovators, providing leading-edge science, sound policy research, and effective program and service development. Keeping abreast of global developments on diseases enabled Health Canada to play a leading role in Canada's response to the SARS, BSE and West Nile Virus outbreaks.
Knowledge Brokers
Through research, risk assessments and surveillance, Health Canada provides knowledge to Canadians and others working in the health care field to enable them to make sound choices to protect health. The Department also monitors and researches the health threats from environmental factors such as toxic substances, air and water pollution, climate change and other threats. This work fosters sound decisionmaking and policy-development at all levels to help reduce health risks.
Enablers
In all program areas, Health Canada brings stakeholders together, as well as provides information, research and education. The work of Health Canada enables Canadians to be up-to-date and informed about the issues that can impact their health.
Trustees/Stewards
Health Canada, through the administration of the Canada Health Act, aims to ensure that all eligible residents of Canada have reasonable access to medically necessary insured services. The Department's broad regulatory responsibilities to protect Canadians and promote health and safety range from prescription drugs and vaccines to toxic substances, from cardiac pacemakers to natural health products and food, from consumer goods to pesticides.
Proponents of Transparency
All work at Health Canada, from the assessment of products under the Canadian Environmental Protection Act to the regulation and approval of thousands of products, is conducted transparently. Health Canada has committed to be accountable in delivering results to Canadians. The public had an opportunity to be involved in consultations on major regulatory initiatives such as the new Pest Control Products Act and will continue to be consulted in other areas as part of the Department's consultations framework.
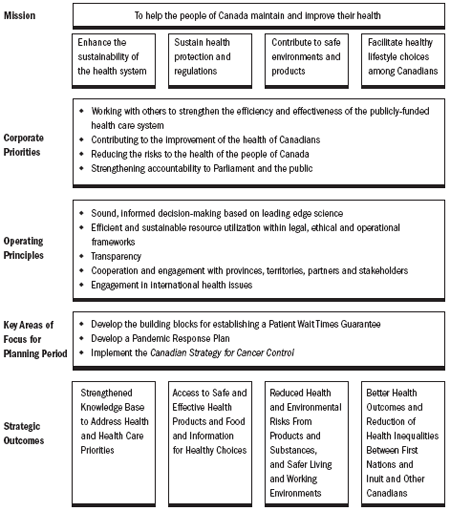
Our Mission and Objectives
Health Canada's mission is to help the people of Canada maintain and improve their health. We strive to accomplish this by promoting and protecting the health of Canadians. In order to achieve this, we will:
- Enhance the sustainability, innovation and integration of the health system;
- Sustain health protection and regulations;
- Contribute to safe environments and products; and
- Facilitate healthy lifestyle choices among Canadians.
Health Canada and partners helping Canadians make independent, informed choices
The responsibility for promoting, protecting and improving the health of Canadians does not rest with a specific level of government, the medical profession or Canadians themselves. The responsibility is found in an interwoven community of collaborating stakeholders that each contributes to this goal.
Canadians make choices everyday that affect their health and well-being. Environmental, economic and social factors also affect health. Municipal, provincial and territorial governments, health service providers and not-for-profit organizations help ensure community health services are available and provide the heath care system that Canadians rely on to protect and improve their health. The private sector helps develop pharmaceuticals and other health products for Canadians.
In addition to overseeing the Canada Health Act, the federal government helps assess risks to human health, sustains health protection efforts, regulates and approves products, and funds health services. Health Canada provides national leadership and expertise in the development of health science and policy. The federal government provides assistance to provincial and territorial governments in the provision of health care services through the Canada Health Transfer.
With respect to health programming and services for First Nations and Inuit, Health Canada supports public health and community health programs on-reserve and in Inuit communities, provides non-insured health benefits coverage regardless of residence, and delivers primary care services in remote and isolated communities to supplement and support the services that provincial, territorial and regional health authorities provide.
Health Canada's Corporate Priorities
Given the environment in which we operate, Health Canada has identified four corporate priorities to respond to the key challenges and opportunities facing the health of Canadians.
These priorities reflect the Government of Canada's direction and commitments as well as our objectives and planned strategic outcomes, which are long-term benefits to Canadians that stem from our overall mission of helping Canadians maintain and improve their health. They represent the differences we wish to make for Canadians. For more information on Health Canada's strategic outcomes, please refer to Section II of this report.
1. Working with others to strengthen the efficiency and effectiveness of the publicly-funded health care system
An efficient and effective health care system is consistently identified as a priority for Canadians. Health Canada will work closely with provincial and territorial governments, as well as health organizations and other stakeholder groups to examine new and innovative ways to strengthen the efficiency and effectiveness of a universally accessible and equitable publicly funded health care system.We will work with the provinces and territories to develop the building blocks for a Patient Wait Times Guarantee to ensure that Canadians receive the care they need, when they need it.
2. Reducing the risks to the health of the people of Canada
The Department plays a core role in protecting and promoting the health and safety of Canadians.
A potential pandemic such as the Avian Flu presents a great risk to Canadians and global health in general. That is why pandemic preparedness is a priority for Health Canada and why we will continue to work with the Public Health Agency of Canada, other countries and the World Health Organization to increase international cooperation efforts. We are already active in contributing to pandemic readiness by working in the areas of vaccines, multilateral contributions, workplace health and safety, and in emergency preparedness within First Nations and Inuit communities.
The health of Canadians is linked to the health of the environment. We are actively developing integrated approaches to better assess impacts on health and to develop strategies to mitigate known and emerging risks from pollutants and toxic chemicals in air, water, food and products, for example. Protecting and improving the health of vulnerable populations, such as children, seniors, and Aboriginal peoples, from pollutants and chemicals will benefit all Canadians. We are also active in developing regulations, which contribute to protecting the health of Canadians as well as managing the risks and benefits of health products and devices. We continue to strengthen scientific capacity to inform our regulatory responsibilities and monitor new developments.
3. Contributing to the improvement of the health of Canadians
While the majority of Canadians enjoy a high quality of life, there are areas for improvement. Health Canada will examine and implement new ways to contribute to the improved health of Canadians through collaborative work with other organizations in the Health Portfolio and with other departments towards the Government of Canada's goal of improving the quality of life of Canadians.We are working with the Public Health Agency of Canada to advance efforts on cancer, to support the efforts of stakeholders and to improve screening and prevention.
Health is more than just physical health. Mental health is an integral part of overall well-being and we are working to address mental health and mental illness issues.
A major area of concern continues to be Aboriginal health. While Aboriginal Canadians are living longer, the status of their health continues to lag behind that of other Canadians. Our goal, in collaboration with Aboriginal organizations, Health Portfolio partners, other departments and the provinces and territories, is to deliver efficient and effective health programs, services and initiatives to help improve health outcomes for First Nations and Inuit people.
4. Strengthening accountability to Parliament and the public
Our ability to effectively respond to the health needs of Canadians depends on rigourous management practices to achieve results and ensure value for money. We are reinforcing our commitment to accountability, transparency and sound management of resources by continuing to integrate the principles of modern comptrollership, introducing improved systems and processes for departmental operations and addressing human resource priorities. As part of our continued effort to strengthen our management practices, we will:
- fortify our management of grants and contributions by ensuring that solid governance structures and administrative processes are in place;
- improve governance and control of contracts using a Contract Management Framework established on the basic principles of responsibility, accountability, monitoring, oversight and audit;
- strengthen accountability and stewardship by improving performance measurement and renewing our program evaluation functions;
- continue to initiate reviews of existing systems and processes in line with Government-wide initiatives;
- continue the implementation of "The Way Forward", an information technology (IT) project that will consolidate and realign IT resources and position the Department to align with Government of Canada common services initiatives and generate savings;
- continue the implementation of the new Chief Financial Officer Branch to support the effective management of resources and improve our ability to achieve results across programs through a strengthened Financial Management and Control Framework; and
- establish a work program to continuously review and improve measurable expected results and performance indicators for the 2007-2008 RPP and beyond.
Health Canada is considered to be a leader within the Government of Canada in implementing the Management Accountability Framework (MAF), a framework that establishes the standards for management accountability in the Government of Canada. Health Canada will continue to build upon this solid foundation by integrating MAF requirements into the management culture of the Department including enhancing a risk-based approach to programs and activities.
Key Areas of Focus for Planning Period
Taking into account the current operating context, the emerging risks to the health of Canadians and the trends in Canadian society, Health Canada will focus on the following key strategic areas for the 2006- 2009 planning period:
- Develop the building blocks for establishing a Patient Wait Times Guarantee - Reach a shared understanding on ensuring that the health care needs of Canadians are met within a universally accessible and equitable health care system. Continue working with provinces, territories and other stakeholders to share best practices and innovative initiatives to develop the building blocks for establishing a Patient Wait Times Guarantee.
- Advance efforts to prepare for a Global Pandemic Outbreak - Collaborating with other international organizations, departments, provinces, territories and stakeholders to ensure that Canada is well positioned to prepare for and respond to a possible pandemic influenza outbreak.
- Implement the Canadian Strategy for Cancer Control - Cancer prevention is a priority for the Government of Canada and Health Canada. To this end, we will collaborate with the Public Health Agency of Canada and other organizations to improve cancer screening, prevention and coordination through the Implementation of the Canadian Strategy for Cancer Control. The Strategy's main objectives are to reduce the number of new cases of cancer in Canada, to enhance the quality of life of those living with the disease and to reduce the number of premature deaths attributable to cancer.
Operating principles
We are guided by several operating principles in the delivery of our programs and services that help us maximize efficiency in reaching our objective of improving and maintaining the health of Canadians. These operating principles cover the broad spectrum of Health Canada's activities, which range from indepth policy analysis to scientific research.
- Sound, informed decision-making based on leading edge science
To bring leadership, coherence and expertise to the overall strategic direction of Health Canada's scientific responsibilities and activities, Health Canada has established the Office of the Chief Scientist (OCS). The OCS will continue to champion science throughout the Department by coordinating involvement in research and regulatory science within Health Canada and within the federal science and technology community, providing scientific expertise on Health Canada priorities, fostering and facilitating partnerships, promoting and communicating Health Canada science and research, and protecting intellectual property.
- Efficient and sustainable resource utilization within legal, ethical and operational frameworks
We are committed to sound financial management and delivering value for money for Canadians. Through the newly established Chief Financial Officer Branch, we will continue to review processes to optimize the effectiveness and efficiency of the use of our resources and follow central agency direction to ensure management accountability.
- Transparency
We strive to develop and deliver our programs and services in an open and transparent manner by ensuring stakeholders and the public have tangible input to our work through vehicles such as public and stakeholder consultation.
- Cooperation and engagement with provinces, territories, partners and stakeholders
We are committed to working with our partners including provincial and territorial governments, First Nations, Inuit and other Aboriginal organizations, communities, professional associations, consumer groups, universities and research institutes, international organizations, not-for-profit organizations, volunteers and other federal departments and agencies.
- Engagement in international health issues
Increased global mobility enhances the quick spread of disease throughout the world and necessitates our active involvement in the international health community. We are committed to learning from the experiences of other countries and their best practices to minimize the risks to Canadians from global health threats.
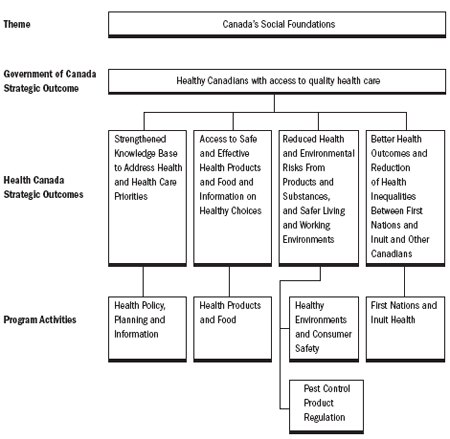
Contributing to Government of Canada Strategic Outcomes
The following chart shows how Health Canada's Program Activities align with the Government of Canada's Strategic Outcomes, which were developed by Treasury Board of Canada Secretariat.
Part B: Internal Areas of Interest for the Planning Period
Health Canada's Operating Environment
Taking stock of domestic and international trends on a variety of aspects and monitoring emerging risks to the health of Canadians help us gain greater insight into Health Canada's operating environment and develop appropriate policies and initiatives. The description of health risks, opportunities and priorities in Canada five years ago does not match today's description and may be very different from what we will face 5 or 10 years from now.
The Changing Face of Canada
Several changes are taking place in Canadian society that affect policy and program development at Health Canada. It is well known that seniors in Canada are growing in number and living longer, posing new challenges for all aspects of health care. The average Canadian child will have an older mother and fewer siblings. A growing proportion of children are living only with single parents and many face low-income. The health system will continue with a growing proportion of them living only with single parents and many still in poverty, especially those living with only their mother. The health system will continue to face the challenges of compromised health and poor living habits among a significant portion of children living in a level of poverty. Over the coming decade, Canadians are more likely to be living in an urban centre. Onefifth will be a visible minority with roots in Asia or the Middle East and speaking languages other than French or English. A more culturally and ethnically diverse society will continue to increase the demand for alternative therapies and service providers.
Technology Pervasive in Daily Life Non-traditional disciplines such as biotechnology, artificial transplants and nanotechnology provide exciting potential to address health issues in new ways and dramatically improve the health of many Canadians in the coming decades. These new technologies will continue to challenge us to have the appropriate regulatory science to input into decision-making.
Ethical and social issues will continue to challenge us in new areas including cloning, DNA manipulation and genomics. As advances in science and technology provide more pervasive solutions in the area of health, it will be imperative to continue to integrate science into decision-making by government, industry and individuals.
The market for pharmaceuticals is expanding at an incredible rate and an efficient and effective health system must respond to this by ensuring that Canadians have timely access to safe and effective health products, drugs, food and information.
Evolving Attitudes and Values towards Health
There has been a significant shift in the Canadian public's perception of health care delivery as they are moving from patients to consumers. Because health information is readily available from a number of different avenues, Canadians are more informed than ever about their health and are more willing to discuss sensitive health issues. Canadians are also seeking new ways to have their health concerns addressed and are influenced by factors, which include religion and culture. They are also more engaged in the review of the public health care system and expect governments to discuss these issues in an open and transparent manner.
Deteriorating Physical Environment
The relationship between human health, the environment and the economy is one of the most complex health areas facing governments in Canada. The health of many Canadians will continue to be threatened by air, water and land pollution, climate change and the thinning ozone layer. The health impacts of many environmental risks are not fully understood. The increasing incidence of respiratory illness from poor air quality could grow with the expansion of urban areas and with the advancement of climate change. The health of Aboriginal communities in the North is especially vulnerable to threats posed by significant changes associated with climate change. Health Canada must be prepared to address the public expectation for protection as well as provide more developed information on the adverse health impacts and enforcement that may be required.
Toward Full Globalization
All departments including Health Canada must acknowledge the increasing unrestricted movement of people, goods and services across the globe and the potential consequences that may arise. Governments and the health community are moving beyond the "what if" to a "when" regarding the possible outbreak of a pandemic. The speed with which a pandemic outbreak can spread internationally is alarming. In order to address this potential disaster, Health Canada is actively participating in and leading many international activities such as the Global Health Security Initiative and the APEC Health Task Force.
Canada's social responsibility to continue to help address the growing health problems faced by the world's poorer nations will only increase as the life-expectancy gap between developed and less developed countries is increased by HIV-AIDS and other new and re-emerging diseases, poverty, child mortality, injuries, and non-communicable diseases.
There are opportunities to help the international community benefit from our experience in many areas including healthy living, tobacco cessation, early childhood health along with disease prevention, vaccinations, and access to safe water, to name a few areas.
Emerging Illnesses, Injuries and Diseases in Canada
While the threat of diseases outside our borders are of particular concern, just as alarming is the growing rate of chronic diseases and injuries in Canada. It is estimated that over 60,000 Canadians will die of cancer this year alone and another 79,000 will die from heart disease. Injuries are the leading cause of death for Canadians aged 1 to 44. What makes these figures particularly alarming is that many of these deaths could be prevented. A more informed population is a healthier one and Health Canada with its partners must work to address these issues and provide Canadians with the information they need to make healthy, independent choices.
Health Canada: Collaboration at Work
At Health Canada, we understand the importance of working collaboratively in order to deliver effective programs and services to Canadians. Working with an integrative and horizontal approach allows us to draw on our strengths and provide effective policy and scientific analysis across our many fields of expertise. For this reason, Health Canada is committed to continuing horizontal collaboration for this planning period. There are many ways in which Health Canada collaborates horizontally with partners to improve and maintain the health of Canadians.
- Departmentally
At Health Canada, we are organized to respond to the various health needs of Canadians. We collaborate internally to provide the best possible services and programs to Canadians. By doing so, we draw upon not only our strengths but our experiences in any given situation. Examples of departmental horizontal initiatives include tobacco and substance abuse programs, environmental health programs, and research on pesticide residues.
- Across the Health Portfolio
Providing health policy leadership and coordination within the Health Portfolio gives Health Canada an important role in the development and implementation of programs and services to Canadians. We collaborate horizontally on a number of health initiatives such as our work with the Public Health Agency of Canada (PHAC) on the Healthy Living and Chronic Diseases Strategy as well as on emergency preparedness and response issues. We obtain much of our health and surveillance data from PHAC. Other examples of cross-cutting initiatives include the alcohol strategy, First Nations and Inuit programs, pandemic preparedness and health benchmarks and indicators.
- Across the Government of Canada
Health Canada recognizes the importance of horizontal initiatives across the Government of Canada. Health Canada is one of the largest departments within the federal government and health is a key consideration in the majority of the government's programs and services. Some of the interdepartmental programs we contribute to include the Canadian Environmental Protection Act and Pesticide Regulation, as well as the Service Improvement Initiative and the Sustainable Development Strategy.
- Provincial, Territorial and Aboriginal
Health Canada works in collaboration with provincial, territorial and Aboriginal organizations on priority areas such as implementing the First Ministers' commitments of the 10-Year Plan to Strengthen Health Care. Ongoing collaborative efforts will include closing the gap in health outcomes between the general Canadian population and First Nations and Inuit; making timely access to quality care a reality for all Canadians; furthering the development and implementation of the National Pharmaceuticals Strategy; and ongoing efforts in public health and pandemic preparedness. We will also undertake a review of commitments of the 2005 Meeting of First Ministers and Aboriginal Leaders.
- Internationally
Health Canada is exploring ways to strengthen the regulatory capacity of developing countries, especially as it relates to imported products, through organizations such as the World Health Organization. We will complete the implementation of the International Regulatory Cooperation Strategic Framework, which will ensure effective prioritization and evaluation of regulatory activities.We will establish a Memorandum of Understanding with the Australian Pesticides and Veterinary Medicines Authority in order to increase international cooperation and information sharing.We will implement regulatory cooperation initiatives under Memoranda of Understanding and Mutual Recognition Agreements on information and technical exchanges with Switzerland, U.S., China and Australia, amongst others. Health Canada is also developing an arrangement with the Therapeutic Goods Administration in the Department of Health and Ageing of Australia that allows for the recognition of quality management systems certificates issued for medical devices.
Responding to Human Resources Risks and Challenges
Health Canada's human resources planning process supports the Department's business objectives.
The Department's Annual Report on Human Resources Indicators identifies human resource management issues, risks and challenges and identifies activities that can be taken to address them. For example, the number of employees who are eligible to retire is rising every year. Therefore, managers have been asked, in their Human Resources planning, to identify succession and knowledge transfer strategies to ensure that the Department can continue to manage staff turnover and deliver results for Canadians.
Given the unique human resources issues at the branch level, each Branch human resources plan identifies the risks and activities to address them. An example of this would be that each Branch has developed a strategy to address the gap in the linguistic capacity of their key feeder groups. As well, given the strong need for renewal in the human resources community the Department is participating in an interdepartmental initiative to recruit and develop qualified human resources professionals. Health Canada is developing a departmental strategic human resources plan that will respond to Branch and Corporate risks, and provide direction for the integrated Human Resources Planning process for 2007-2008.
As a science-based department, Health Canada hires scientists as researchers and regulators, in healthrelated fields and in pure and applied science. The Department has identified several science specialties as 'shortage areas', and has developed a recruitment strategy and an employment inventory to ensure there is a pool of candidates available to fill vacant positions.
In addition, the Department performs a regular workforce analysis to identify gaps in employment equity representation (women, Aboriginal people, persons with disabilities and members of visible minority groups) and to identify measures that can be taken to address those gaps. As a result, since April 2, 2004, the representation of employees from employment equity groups has met or exceeded the proportion of such individuals available from the labour force.
Finally, in 2006-2007, we will continue to support the implementation of the Public Service Modernization Act (PSMA), a major building block in the Government of Canada's overall strategy to modernize human resources management through training and communication activities, the review of staffing policies and guidelines, the piloting of new staffing tools and approaches, and the implementation of a Staffing Monitoring Action Plan. The PSMA and the strengthening of corporate services through the human resources planning process will help ensure that the Department has the human resources it requires to deliver on its mission.
Incorporating Sustainable Development Principles into Practice
Health Canada will continue to work towards fulfilling departmental commitments outlined in its Sustainable Development Strategy 2004-2007, Becoming the Change We Wish to See, in which programs and services identify how they will incorporate sustainable development principles into practice.
Further exploration of the social dimension and its impact on health will be undertaken to better integrate this pillar with environmental and economic pillars within the context of the development of the Sustainable Development Strategy 2007-2010.
In the upcoming year, efforts will be made to work across federal departments and create interdepartmental targets, where appropriate, to facilitate better linkages of activities fostering a sustainable development approach in areas of mutual interest. Health Canada will also do its part to contribute towards government-wide initiatives, including integrating green procurement policy into the Department.
Endnotes:
1 For more information on Legislative Acts, please visit the Department of Justice Canada's
website at: laws.justice.gc.ca/en/index.html
2 www.phac-aspc.gc.ca/new-eng.html
3 www.cihr-irsc.gc.ca/
4 www.hmirc-ccrmd.gc.ca/
5 www.pmprb-cepmb.gc.ca/
6 www.hc-sc.gc.ca/hl-vs/reprod/agenc/index-eng.html
Section 2: Analysis of Program Activities by Strategic Outcome
Strengthened Knowledge Base to Address Health and Health Care Priorities
| This program activity contributes to the Government of Canada Strategic Outcome: Healthy Canadians with Access to Quality Health Care. | ||||
| Planned Spending and Full-Time Equivalents (FTEs) | ||||
|---|---|---|---|---|
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
| Net expenditures | 375.1 | 288.4 | 218.2 | 215.0 |
| FTEs | 717 | 627 | 604 | 588 |
|
Notes: The decrease in expenditures from 2005-2006 to 2006-2007 is mainly due to a decrease in the level of funding of the Primary Health Care Transition Fund, the sunset of the Northern Health Supplement to the 2003 Health Accord, and the Expenditure Review Committee (ERC) reduction. The decrease in expenditures from 2006-2007 to 2007-2008 is mainly due to the sunset of the Primary Health Care Transition Fund. The decrease in expenditures from 2007-2008 to 2008-2009 is mainly due to a decrease in funding for the Implementation of Health Canada's Therapeutic Access Strategy. Figures include an amount for other departmental and regional infrastructure costs supporting program delivery. |
||||
Program Activity Description
The objective of this program activity is to provide policy advice and support to the Minister in making decisions to protect and improve the health of Canadians. Health Canada supports the delivery of programs and services to Canadians by developing policies and building and maintaining linkages with other partners to support health care system reform. We also work with international organizations to advance a global health agenda and contribute Canadian expertise. This helps to ensure the health, safety and security of Canadians in a healthier world. We provide a leadership role in strategic planning for the Department. We administer the Canada Health Act, and work with provinces and territories on health care renewal and support. We work with others to provide access to health care services for official language minority communities, and the interface between different sectors of the health care system.
To ensure that all Canadians have access to health services when and where they need them, that the quality of those services is continually improved, and that the system can provide the necessary care today and has the capacity to identify and adapt to the emerging needs and challenges of tomorrow, we are focussing renewal efforts, amongst other things, on the health human resources.
Another type of renewal effort is in the legislative and regulatory arenas. Legislation and Regulatory Renewal is an opportunity to deliver a much anticipated, significant and modernized legislative framework for the Health Portfolio. Much of the health protection legislation that forms Health Canada's regulatory base is out of date and not in line with modern technological advancements or public expectations, leading to gaps in what is covered, inconsistencies in addressing health risks and inadequate enforcement/compliance powers.
We provide policy advice and lead initiatives to advance women's health and to increase understanding of how gender interacts with the other determinants of health to affect health outcomes of women, men, boys and girls over their lifespan.
We undertake research and analysis to improve the availability, quality and use of evidence in health policy decision-making. We reach our goal by identifying future policy research needs, conducting extramural peer-reviewed policy research to meet these needs, communicating the results within Health Canada and externally, and by providing the expertise and tools needed for a sound and rigorous analysis of health policy options.
Our priorities
In addition to tracking emerging issues on an ongoing basis, we continue to actively participate in and collect invaluable information from various scanning activities to help identify future risks to Canadians and challenges to Health Canada. It is important to note that numerous external factors can influence our ability to focus exclusively on our priorities (e.g., the increased attention to global preparedness and response to a possible pandemic influenza outbreak or coordinating relief efforts for natural disasters). We intend to focus on the following priorities in 2006-2007:
Partner in health reform
In the 2004 Health Accord, federal, provincial and territorial governments committed to health system reforms that will improve
timely access to quality care. To support the Accord, the federal government is flowing $41 billion to provinces and territories
over ten years, including $5.5 billion to augment provincial/territorial existing investments and efforts in wait times reduction.
In December 2005, provincial and territorial governments announced a first set of ten common evidence-based benchmarks in the
areas such as cancer screening and care, cardiac surgery, hip and knee replacements and cataracts. Health Canada will work with
the provinces and territories on the development of a Patient Wait Times Guarantee. Care guarantees have been suggested by many
experts as one of the measures to reduce wait times.
We have made progress on all initiatives in the Accord and all governments are moving forward with their health system reforms.Work is also continuing to implement 2004 Health Accord initiatives in the following areas:
- implementing the Internationally Educated Health Care Professionals (IEHP) Initiative, which will provide additional funds to accelerate and expand the assessment and integration of IEHPs for participating governments;
- monitoring the commitment to provide first-dollar coverage by 2006 for certain home care services, based on assessed need, including: two week provision of case management and intravenous medications related to discharge diagnosis, nursing and personal care for short-term acute home care; two-week provision of case management and crisis response services for short-term acute community mental health home care; and case management, nursing, palliative-specific pharmaceuticals and personal services for end-of-life care;
- working to ensure that the populations served by federal departments (specifically First Nations, Inuit and veterans) will have access to the home care services specified in the Accord;
- continuing to support the Best Practices Network for primary health care, which is facilitating information sharing and addressing common barriers to progress; and
- working with the Health Council whose mandate is to monitor and make annual public reports on the implementation of the 2003 First Ministers' Accord on Health Care Renewal and to report on progress of the elements set out in the 2004 Health Accord.
In 2006-2007, we will continue to collaborate with our provincial and territorial counterparts as we implement commitments to health care system reform. 1
The Primary Health Care Transition Fund (PHCTF) ($800 million over six years) is providing funding to the provinces and territories to support their efforts in reforming their primary health care systems. One of the objectives of the primary health care reform is to strengthen health promotion and prevention activities (both primary and secondary) within the sector so it can help Canadians make healthy lifestyles choices and thereby reduce the incidence of conditions such as diabetes and cancer. With PHCTF-funded initiatives concluding in 2006-2007, dissemination activities are planned to promote the uptake of knowledge and results. In turn, these knowledge transfer activities will support ongoing reform activities.
In concert with other departments involved in the Action Plan on Official Languages, we will work towards implementing administrative practices and policies to ensure that the enhanced accountability provisions of the Official Languages Act, which were introduced in November 2005, will be reflected in the provision of health services to official language minority communities across Canada.
Hepatitis C
The Government of Canada is committed to helping all those infected with hepatitis C. On July 25, 2006, the Prime Minister
announced that the government reached an agreement on the elements of a settlement for those Canadians who contracted hepatitis C
from the blood system before January 1, 1986 and after July 1, 1990.
Under the terms of the agreement, the Government of Canada will set aside nearly $1 billion in a special settlement fund. The level of compensation will be based on the principle of parity with compensation already provided by the federal government for those who were infected between 1986 and 1990.
Benefits will be paid on a present-value basis, meaning that class members will receive the entire sum of their compensation up front, based on such factors as current disease level and probability of disease progression. This will also serve to minimize administrative costs.
The Government of Canada will be working as quickly as possible to complete the steps needed before compensation is provided to the class. A final detailed agreement needs to be completed, and must be approved by Courts in four jurisdictions. Furthermore, an administrative structure must be set up to evaluate applications and forward payments. The federal government cannot control the timing of every remaining step, however, all efforts will be made to ensure that this proceeds as quickly and as effectively as possible.
Pandemic Influenza
Health security is a critical component of Canada's objectives for health policy, foreign policy and national security. The most
pressing challenge for health security at the current time is the threat of an influenza pandemic. As such, it is critical that
the Government of Canada is prepared for an influenza pandemic, and that work is undertaken with partners in the public and
private sectors domestically and internationally to strengthen preparedness throughout Canada.
The potential severity and impacts have resulted in unprecedented co-operation and collaboration on a global health issue. We will continue to play an active role in preventing and preparing for avian and human pandemic influenza. This involves close collaboration with the Public Health Agency of Canada, which is the public health lead for pandemic influenza preparedness in the Government of Canada. We will strengthen preparedness in the Department's key areas of responsibility, such as First Nations and Inuit health, the regulation of vaccines, and occupational health services for federal employees. We will also complete a business continuity plan in the event of an influenza pandemic to ensure that support is provided for employees and that critical services can continue to be delivered in the event of large-scale worker absenteeism.
We will also focus on international collaboration for avian and human pandemic influenza preparedness and response, which is a critical element of Canada's domestic preparedness. Governments across the world have declared avian influenza to be a "global threat", and have recognized that international collaboration is necessary in order to control the H5N1 avian influenza outbreak, and to prepare for an influenza pandemic. Intensive efforts are underway through multilateral organizations (e.g., World Health Organization, Food and Agricultural Organization, World Organization for Animal Health) and through regional organizations (e.g., Asia Pacific Economic Cooperation, the Security and Prosperity Partnership in North America) and other fora such as G8 to collaborate in preparedness efforts.
Mental Health
Mental health and well-being are fundamental to Canadian's quality of life, as well as our social and economic development. At the
same time, mental illnesses such as depression, anxiety disorders, schizophrenia and bi-polar disorders represent a significant
public health challenge, impacting as many as 1 in 5 Canadians and resulting in significant costs to the health care system,
society and the economy.
In May 2006, the Standing Senate Committee on Social Affairs, Science and Technology released a report on mental health, mental illness and addiction in Canada. Entitled Out of the Shadows at Last, the report underscored the breadth of the challenge associated with mental health, mental illness and addiction, as well as the need for governments to work together in addressing this important issue. Canada is currently the only G7 country without a national strategy or action plan on mental health. The federal government will work with its partners to build the foundation for a national approach to mental health and mental illness in Canada.
Health Canada will also continue to support the development of sound mental health policies and programs within the federal government, and among the provinces and territories. This includes ongoing improvements to the mental health programs, services and support to First Nations and Inuit, as well as broader efforts to improve the mental health and well-being of all Canadians, in areas such as research, information and knowledge exchange, and best practices.
Pharmaceuticals Management Strategy
Drug therapy is an increasingly important component of modern health care. Appropriately prescribed and used, pharmaceuticals can
improve health outcomes for individuals and reduce costs in other health care sectors, e.g., hospitals. The development of new
drugs has the potential for even greater benefits in the future. Despite their benefits, prescription drugs pose a number of
challenges related to equitable and affordable drug access, drug safety and effectiveness, optimal drug therapy, and health care
system sustainability. 2
Health Canada has a number of roles with respect to pharmaceuticals at different points in the drug life cycle, including at the research and development, market approval, prescribing, access, utilization and reimbursement stages. In support of improved health outcomes for Canadians and system sustainability, we will work to optimize these roles using available policy, regulatory and program instruments to better integrate pharmaceuticals into a seamless, robust health care system. We will seek to capitalize on opportunities in areas such as post-market drug safety and effectiveness, appropriate drug prescribing and use, and the drug pricing and research role of the Patented Medicine Prices Review Board.
We will also continue to work with the provinces and territories on pharmaceutical activities initiated as part of the 2004 Health Accord under the National Pharmaceuticals Strategy -- an integrated, collaborative, multi-pronged approach to addressing pharmaceutical challenges that builds on governments' shared roles in the pharmaceuticals sector and previous collaborative pharmaceutical initiatives. These activities will be linked, where appropriate, to federal initiatives to modernize the regulatory system for therapeutic products and to integrated pharmaceuticals management among federal jurisdiction drug plans.
Legislative Renewal and Regulatory Reform
Under the Health Protection Legislative Renewal exercise, which responds to shortcomings in Health Canada's legislative basis for
health protection, Health Canada, with the Public Health Agency of Canada, continues to review its health protection legislation.
The review is intended to modernize and reinforce key existing legislation, namely the Food and Drugs Act (1953), the Hazardous
Products Act (1969) and the Radiation Emitting Devices Act (1969) through the development of enhanced health
protection legislation. The resulting legislative framework will serve to modernize and strengthen the existing federal laws
dealing with health protection and provide clear policy direction. As part of this exercise, the Department is also engaged in
reviewing the proposed legislation to determine whether to proceed with a single piece of legislation or to continue with a phased
approach as started by the expediting of the new Quarantine Act (2005).
Under the auspices of the Government of Canada's Smart Regulation initiative, Health Canada has been actively contributing to the development of a series of policies, frameworks and tools aimed at modernizing the Canadian regulatory system so that it can better respond to the challenges it currently faces (e.g., rapid scientific developments, globalization, or cross-boundary health risks, etc.) The goal of the initiative is to build a robust and flexible regulatory system that not only maximizes health, safety and environmental protection but also promotes an innovative economy.
This year, we will continue to coordinate the Department's input into a proposed Government Directive on Regulating (GDR), which is intended to build on the existing Federal Regulatory Policy by promoting increased regulatory transparency, the alignment of legislative and regulatory planning, and strategic coordination and collaboration with provincial, territorial and international partners. 3
Establishment of the new Assisted Human Reproduction Agency of Canada and new regulations
We will continue to work toward the implementation of the Assisted Human Reproduction Act, including support to the Assisted Human
Reproduction Agency's successful establishment in Vancouver, B.C. in 2006-2007. The Agency will license and inspect activities
controlled under the Act. The recruitment process leading to Governor in Council appointments to the Agency's new board of
directors will be completed.
We are proceeding concurrently with the development of the components of the regulatory framework, which are required before the Agency can implement the licensing and regulatory regime for activities controlled under the Act. The fact that very little currently exists in terms of established guidelines, standards or regulations necessitates careful and comprehensive consultations, to ensure that regulatory objectives are met while at the same time minimizing the regulatory burden on Canadians. Regulations are expected to be promulgated in 2006-2007 to bring the last outstanding prohibition into effect and work will continue to develop the remaining components of the regulatory framework to implement the Act.
Health Human Resources
The health care sector is labour intensive. Between 60 and 80 cents of every health care dollar in Canada is spent on health human
resources (HHR), and this does not include the costs of education. Currently, there are reported shortages for physicians, nurses
and other health care providers. HHR is one of the four cornerstones to support real health system change. Therefore, building
capacity in the system and providing adequate supply, distribution, and appropriate use of HHR is critical to reducing wait times
and improving timely access to health care.
Building on health human resource activities that support the 2003 Accord and the 2004 Ten-Year Plan, we will continue with the implementation of the Health Human Resource Strategy through three broad initiatives (Pan-Canadian Health Human Resource Planning; Interprofessional Education for Collaborative Patient-Centred Practice; and Recruitment and Retention) and the Internationally Educated Health Care Professionals (IEHP) Initiative, which will provide additional funds to increase health care professional supply through the acceleration and expansion of the assessment and integration of IEHPs for participating governments.
Role of science
It is through scientific discoveries and innovations that the greatest potential benefits for the health of Canadians lie. Science also provides a foundation of evidence for policies and programs to improve the health of Canadians. In addition to work in many health sciences policy areas, we will focus on the following areas in 2006-2007:
- The dramatic increase in the development and use of genetic technologies in the health system has clear implications for the sustainability of Canada's health care system in terms of potential new ways to prevent, diagnose, treat and cure thousands of conditions. The development of new genomic-based drugs (pharmacogenomics) and diagnostics will also affect health care delivery. We will undertake policy research and analysis to support federal discussions and action in both domestic and international fora on issues such as pharmacogenomics, patent pooling in medical genetics, intellectual property issues related to stem cell research, and the development and uptake of OECD guidelines on quality assurance in genetic testing. We will also explore approaches to stimulate innovation and undertake analysis of various incentive mechanisms to spur research and development to address health needs, including vaccine development.
We continue to be committed to working with our partners on early issue identification and the monitoring of emerging technologies that impact the health of individuals, vulnerable populations and the overall health system. We will support the development of evidence-based recommendations and strategies through continued collaborative work to support excellence in the ethical conduct of human research in areas such as: research ethics education, quality improvement, the examination of models of accreditation and the development of process standards for research ethics boards; policy development on good research practices related to biobanking of human biological material samples; and the identification of potential societal impacts of nanotechnology. 4
Human resources risks and challenges
The human resources challenges are very similar to those faced in other areas of the Department and even across the federal government including staff turnover, retention and the recruitment of skilled and knowledgeable policy analysts. Given the extent of our involvement in policy work and the importance of a viable policy capacity, these issues must be addressed to ensure we maintain an adequate capacity to address future policy issues. Though we are heavily involved in recruitment programs (e.g., Economist and Sociologist Development Program), we continue to face challenges in recruiting and retaining policy analysts, given the competitive labour market in this area.
Horizontal linkages
We continue to manage and collaborate on horizontal files for the Department and build linkages within and outside of Health Canada. For example, we manage and coordinate the Federal Inter-departmental Task Force on Mental Health.We also work across the Health Portfolio to ensure that public health is integrated within our advice and support to the Minister.
Continuing on the horizontal policy research themes (Health Innovation, Research on Regulation, Healthy Communities and First Nations and Inuit Health Sustainability) that resulted from the policy research priority setting exercise completed in 2004-2005, cross Portfolio steering committees pertaining to each of the four priority themes were established under Memoranda of Understanding (MOUs) among our branches and the Public Health Agency of Canada. These MOUs will guide our research efforts over the next two to four years.
Since February 2004, the federal Interdepartmental Working Group on Trafficking in Persons, including organs and tissues (IWG TIP), with 14 federal departments and co-chaired by the Departments of Foreign Affairs and Justice, was mandated to coordinate federal efforts to address TIP and develop a federal strategy.We are the lead for the Health Portfolio and we continue to coordinate the Portfolio's input while promoting a holistic approach to the inclusion of health, gender and diversity considerations.
Performance Measurement Strategy
We continue to enhance our performance measurement to provide information on our activities. To streamline the numerous reporting mechanisms, we adopted a new strategy, linking our activities directly to outcomes over the immediate (one to three years), intermediate (three to five years) and long term for outcomes five years and beyond. This approach will support all of our public reporting requirements including the Program Activity Architecture and the Departmental Performance Report. During this reporting cycle, we will work towards collecting performance information to report on the period over the next three years.
The intermediate outcomes planned for the policy planning and research program activity identified below will be addressed in various areas. As a result, a wide spectrum of activities across the Portfolio will be directed towards the same goal.
| Expected Results | Performance Indicators |
|---|---|
| Goals and objectives identified for specific strategies and initiatives |
|
| Knowledge development and transfer of specific health policy issues |
|
Key Programs and Services
Health Care System Policy
In September 2000, First Ministers agreed to continue to make primary health care reform a priority and indicated that improvements to primary health care are crucial to the renewal of health services. In response to this commitment, the Government of Canada announced the Primary Health Care Transition Fund (PHCTF), an $800 million investment from 2000-2001 to 2006-2007. The Fund is supporting provinces, territories and various health care system stakeholders, via contribution agreements, in their efforts to reform the primary health care system. More specifically, it will support the transitional costs of implementing sustainable, large-scale primary health care renewal initiatives which will improve access, quality of care, accountability, and integration of services. Although the PHCTF itself is time-limited, the main goal of the Fund is to bring about permanent and sustainable changes to the organization, funding and delivery of primary health care services. The fund is supported by a federal, provincial and territorial advisory group and Health Canada plays an active facilitation role to foster dialogue and knowledge sharing between and among recipients (government and non-government). With PHCTF-funded initiatives concluding in 2006-2007, all recipients will be submitting final reports including their evaluation reports, and dissemination activities are planned to promote the uptake of knowledge and results. In 2004-2005 a mid-term evaluation of the PHCTF was conducted to assess program structure and effectiveness. The final, or summative evaluation, will be conducted in 2006-2007 and will focus on the results and impact of the PHCTF; it will be available in early 2007-2008.
Health Canada is also supporting knowledge transfer and the uptake of successful strategies the Best Practices Network (FMM 2004). The Primary Health Care Transition Fund will ensure the results and lessons learned of the program are shared for continued progress in primary health care renewal. Planned activities include:
- a series of synthesis papers highlighting the evidence in areas such as chronic disease management, information management, collaborative care (i.e. teams) and evaluation methodology;
- a series of fact sheets on the results and evidence of each initiative;
- a national conference in February 2007; and
- a database, accessible via Health Canada website, to be an ongoing resource for provinces/territories, stakeholders and the public.
Several provincial and territorial governments have publicly committed to continuing the primary health care reforms begun under the PHCTF.
Recognizing the evidence gap for quantitative data on primary health care in Canada, the PHCTF funded the Canadian Institute of Health Information to develop a set of consensus-based national indicators. A legacy of the PHCTF will be the improved capacity for evaluating primary health care. As well, the Health Council of Canada's monitors and reports publicly on primary health care progress.
Note: With the exception of Quebec, all provinces and territories (and in fact all recipients) are required to submit regular reports to Health Canada including narrative progress reports, financial reports, final reports on results and final evaluation reports. These requirements are consistent with the Treasury Board Transfer Payment Policy and related accountability requirements associated with contribution agreements.
| Expected Results | Performance Indicators |
|---|---|
| Knowledge development and transfer of specific health policy issues |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 66.1 | 20 | 0.1 | 0 | 0.1 | 0 |
| *All financial figures in millions of dollars | |||||
Intergovernmental
Health Canada is responsible for the administration of the Canada Health Act (CHA), which involves monitoring a broad range of sources to assess provincial and territorial compliance with the criteria and conditions of the Act, working in partnership with provinces and territories to investigate and resolve CHA compliance issues, pursuing activities to encourage provincial and territorial compliance with the CHA, providing policy advice and informing the Minister of possible non-compliance with the Act, and recommending appropriate action.
The Department also provides strategic advice and coordination on a full range of Health Portfolio policy issues involving collaboration with provincial and territorial partners, while ensuring that federal priorities are advanced.
Under the Official Languages Act and the Action Plan for Official Languages, we manage health contribution programs to support the vitality of official language minority communities across Canada and ensure ongoing consultations with these communities.
| Expected Results | Performance Indicators |
|---|---|
| Knowledge development and transfer of specific health policy issues |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 44.0 | 64 | 29.8 | 64 | 29.8 | 64 |
| *All financial figures in millions of dollars | |||||
International
Public health risks and threats originating beyond Canada's borders increasingly influence the health of Canadians. International collaboration on global health policies and developments is of growing importance to the sustainability and responsiveness of Canada's health system. Health Canada positions itself internationally to: anticipate and respond to international health developments and their impact on Canadians and the health system; influence international health events and fora to improve health globally; provide leadership on selected health issues such as pandemic preparedness, HIV/AIDS and tobacco; and work with the multiplicity of players on the global health scene to advance health and health security.
In order to shape and strengthen the international agenda on health and health care issues, consistent with Canada's priorities and values, we will continue to work in close cooperation with multilateral agencies such as the World Health Organization (WHO) and the Pan American Health Organization (PAHO).
| Expected Results | Performance Indicators |
|---|---|
| Knowledge development and transfer of specific health policy issues |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 21.8 | 26 | 21.6 | 26 | 22.0 | 26 |
| *All financial figures in millions of dollars | |||||
Assisted Human Reproduction Implementation Office
Description: Set-up of Assisted Human Reproduction Agency of Canada
| Expected Results | Performance Indicators |
|---|---|
|
The Assisted Human Reproduction (AHR) Agency begins operations in 2006-2007. Regulatory development - Consultations to continue on all remaining sections of the AHR Act in 2006-2007 to be followed by drafting of regulations in preparation for prepublication in Canada Gazette Part I. Progress towards the development of the Personal Health Information Registry. |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 4.9 | 29.8 | 3.3 | 22 | 1.6 | 8 |
| *All financial figures in millions of dollars | |||||
Legislation Renewal
Health Canada will continue the development of a proposal to replace the Food and Drugs Act(1953), parts of the Hazardous Products Act (1969/safety of consumer and industrial products) and the Radiation Emitting Devices Act (1970), with a new Health Protection Legislative framework. The objective of the new framework is to update, strengthen, and integrate federal health protection legislation to be more responsive to present and future social and technological realities and provide the tools needed to better protect the health and safety of Canadians.
| Expected Results | Performance Indicators |
|---|---|
|
Goals and objectives identified for specific strategies and initiatives |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 1.3 | 0 | 1.3 | 0 | 1.3 | 0 |
| *All financial figures in millions of dollars | |||||
Women's Health and Gender Analysis
Health Canada works horizontally to promote equitable health outcomes across the lifespan for women, men, boys and girls. It provides leadership and oversight to: women's health; women's health research and information; gender based analysis; and, with a diversity overlay, in policy development within the Health Portfolio. It funds the Centres of Excellence for Women's Health, Canadian Women's Health Network as well as Research Working Groups, and collaborates with an expansive network of women's health organizations and other stakeholders at the international, provincial and regional levels to engage the public in the policy development process.
| Expected Results | Performance Indicators |
|---|---|
|
Knowledge development and transfer for specific health policy issues Enhanced health policy research capacity (ongoing) |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 4.9 | 19 | 4.9 | 19 | 4.9 | 19 |
| *All financial figures in millions of dollars | |||||
Applied Research, Dissemination and Accountability
In the area of applied research and analysis, we support policy decision-making by developing and implementing a strategic policy research agenda for medium and long-term issues, helping co-ordinate internal and external policy research activities, and funding extramural research under the Health Policy Research Program. This fosters a performance-based and outcomeoriented culture by developing the tools and information base for better accountability. It plays a key role in knowledge management by managing a policy research dissemination program, including publication of the Health Policy Research Bulletin, and by making core data sets and the informatics tools to access them available.
| Expected Results | Performance Indicators |
|---|---|
|
Target audiences accessing data, analysis and information that is useful for evidence-based policy and program development |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 4.9 | 60 | 36.6 | 60 | 36.6 | 60 |
| *All financial figures in millions of dollars | |||||
Endnotes
1 www.hc-sc.gc.ca/hcs-sss/delivery-prestation/fptcollab/2004-fmm-rpm/index_e.html
2 For more information on these challenges, see the National Pharmaceuticals Strategy Progress
Report, summer 2006.
3 www.regulation.gc.ca/default.asp?Language=E& Page=thegovernementdirectiveon
4 Nanotechnology is defined as the application of science to develop new materials and products,
where at least one dimension is smaller than 100 nm, and involves the manipulation of matter at the nanometre scale - a nanometre
(nm) is a billionth of a metre (10-9m), or a hundred times smaller than a virus.
Other programs and services that contribute to this program activity total $140.5 million for 2006-2007.
Strategic Outcome: Access to Safe and Effective Health Products and Food and Information for Healthy Choices
Program Activity - Health Products and Food
This program activity contributes to the Government of Canada Strategic Outcome: Healthy Canadians with Access to Quality Health Care.
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
|---|---|---|---|---|
| Gross expenditures | 294.6 | 303.3 | 300.4 | 289.3 |
| Less: Expected respendable revenues | 37.7 | 41.2 | 41.2 | 41.2 |
| Net expenditures | 256.9 | 262.1 | 259.2 | 248.1 |
| FTEs | 2,503 | 2,592 | 2,667 | 2,656 |
|
Notes: The increase in expenditures from 2005-2006 to 2006-2007 is mainly due to an increase in the level of funding for Strengthening the Safety of Drugs, and is partially offset by a decrease in funding for the Implementation of Health Canada's Therapeutic Access Strategy and the Expenditure Review Committee (ERC) reduction. The decrease in expenditures from 2006-2007 to 2007-2008 is mainly due to the sunset of funding for Health Canada's Response to Bovine Spongiform Encephalopathy in Areas of Risk Management and Targeted Research. The decrease in expenditures from 2007-2008 to 2008-2009 is mainly due to decrease in funding for the Implementation of Health Canada's Therapeutic Access Strategy, and the sunset of a transfer from Agri-Food and Agriculture Canada for the Agriculture Policy Framework. The change in the FTEs is due to the increase of the salary component of the operating budget. Figures include an amount for other departmental and regional infrastructure costs supporting program delivery. |
||||
Program Activity Description
As Canada's federal authority responsible for the regulation of health products and food, Health Canada evaluates and monitors the safety, quality and effectiveness of the thousands of drugs, vaccines, medical devices, natural health products and other therapeutic products available to Canadians, as well as the safety and nutritional quality of their food. Under this program activity we also review veterinary drugs sold in Canada for safety and effectiveness for animals, and for the safety of foods derived from animals treated with these drugs. Finally, we promote the health and well being of Canadians through a broad range of activities related to health products and food, including developing nutrition policies and standards such as Canada's Food Guide to Healthy Eating.
A core federal health protection responsibility is the regulation of therapeutic products under the legislative framework of the Food and Drugs Act. The federal government's role in protecting health and safety is well recognized and supported by Canadians. However, the regulatory system for drugs and other therapeutic products is facing a number of challenges, including: rapidly advancing science and technology; public expectations for access, safety and transparency; improving linkages to health system decision-making regarding coverage for, and cost of, pharmaceuticals and related products in current systems; and meeting industry demands for faster approvals and increased intellectual property protection.
There is strong evidence of rising rates of acute and chronic disease directly associated with diet (e.g., diabetes among Canadians). While Canadians are increasingly aware of the threats to their health from factors like poor diet, physical inactivity and excessive weights, work and leisure patterns in our society have changed, making us less active and more reliant on foods high in energy which is contributing to increasing rates of chronic disease. With respect to food safety, emergence of new foodborne pathogens and emerging infectious diseases (e.g., avian influenza), and the threat of bioterrorism (e.g., linked to botulism), have increased consumer expectations in Canada and abroad for the role of governments in ensuring the quality and safety of Canada's food supply.
In moving forward, there is a need to continue to modernize legislative and regulatory frameworks to keep pace with changing science, consumer expectations, international developments and other pressures for change. To ensure an integrated approach, it is suggested that a long-term plan to modernize the regulatory system for therapeutic products be developed, based on a vision to improve access to safe, effective and affordable drugs and other therapeutic products. To strengthen collaboration with provincial and territorial governments in their health system and drug plan management roles, this plan needs to be strongly linked to the National Pharmaceuticals Strategy. From the perspective of food and nutrition, Health Canada will continue to work with Health Portfolio partners, the Canadian Food Inspection Agency and other partners to provide leadership on food policy issues in order to improve health outcomes for Canadians and reduce the burden on the health care system.
Health Canada relies heavily on science and risk management in making our regulatory and policy decisions. As scientific knowledge and technical expertise are critical inputs into the development of our regulations, policies and advice, we will continue to conduct laboratory-based research. We will focus on the human health implications of potentially hazardous chemicals in the food supply, including effects on behaviour and the immune system; conditions leading to the survival, growth and toxin production of infectious and toxigenic bacteria; awareness of hazard-prone foods; establishment of safe levels to prevent human injury; and risks and benefits associated with nutrients in the diet. We will also continue to conduct research in areas related to the safety and effectiveness of biotherapeutics, blood and blood products, and vaccines.
Health Canada faces a pressing challenge in sustaining our human resources. It is estimated that within the next five years a significant number of our employees in the specialized and technical fields, which includes biologists, chemists and medical officers, will be eligible for retirement.We will continue to address this through our human resources planning.
We are working with federal science and technology partners to promote and protect the health and safety of Canadians. Some of our work includes collaborating directly with Agriculture and Agri-Food Canada, Environment Canada, the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC) to develop responsive food policies and regulations, such as Canada's Strategy for Safe Food. We are also working with CFIA to promote food safety as a science priority for Canada. Our ongoing work with Statistics Canada and PHAC is enabling us to collaborate on public health data surveys as well as to share information that is important in our ongoing analysis of and preparation for potential health risks associated with emerging diseases.
Internationally, Health Canada is working with the World Health Organization (WHO) and other multilateral organizations on health product and food safety issues. We are also working with other governments to strengthen and promote broader regulatory cooperation and encourage technical information exchange. We will continue to implement the commitments in the Security and Prosperity Partnership of North America, including hosting discussions on pharmaceutical review processes, food safety regulatory coordination, assessment and enhanced surveillance research with related agencies in Canada, the U.S. and Mexico. Bilaterally, we will work with the U.S. Food and Drug Administration through our memorandum of understanding, and on initiatives such as the development of a single set of reference values for nutrition labelling and improving the compatibility between our approaches to food fortification.
Health Canada's regional offices contribute to the delivery of our mandate by developing and delivering key programs and services, including monitoring risks, and the investigation and inspection associated with the importation, sale and manufacture of health products. Working directly with regional stakeholders and provincial and municipal governments, regional offices promote and facilitate consultation and collaboration.1 These partnerships are allowing our regional offices to participate in the monitoring of adverse reactions and assist in developing policy responses on food safety, nutrition, natural health products, antimicrobial resistance, and the efficacy of health products. Further, regional laboratories are increasing our science capacity to develop and manage food safety research and surveillance projects on natural toxins, genetically modified food, food allergen detection, method development and nutrition as part of the national laboratory network. Two of these laboratories are also supporting our responsibilities to ensure manufacturer compliance with regulatory requirements for health products.
Performance Measurement Strategy
The Performance Measurement Strategy for this strategic outcome will help us measure our expected results over time, and determine if our current program activity is appropriate to ensure Canadians have access to safe and effective health products and food, as well as to provide useful information for healthy choices.
| Expected Results | Performance Indicators |
|---|---|
| Access to Safe and Effective Health Products and Food and Information for Healthy Choices |
|
Key Programs and Services
Pre-market evaluation and regulatory process improvement
Description: Making regulatory functions more efficient, effective, and responsive to Canadians by streamlining processes and collaborating more closely with other organizations to ensure Canada continues to have a world class regulatory environment.
| Expected Results | Performance Indicators |
|---|---|
|
Improved timeliness, transparency and predictability of the regulatory process |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 125.8 | 1,164.3 | 124.4 | 1,198.0 | 119.1 | 1,193.1 |
|
* Data is collected and reported quarterly. Our commitment is to meet 90% of performance targets for new pharmaceutical drug submission reviews by March 2006, and for new biologic drug submissions by March 2007. Baseline data for expected performance results is available within current tracking systems and internal records. ** All financial figures in millions of dollars |
|||||
Health Canada will contribute to regulatory renewal through improving regulatory performance and modernizing the regulatory system. 2 For example, given the increasing number and complexity of drug submissions, the initiatives under the Therapeutics Access Strategy will continue to reduce and eliminate submission backlogs, enabling us to meet our performance targets for pharmaceuticals in March 2006 and biologics and genetic therapies by March 2007. 3 In addition, we are reducing submission times for veterinary drugs and have set service standards dependent on submission type. These milestones will be met by enhancing human resources capacity and increasing international collaboration and cooperation as well as adopting and applying project management techniques. The Community of Federal Regulators, a partnership of all federal departments and agencies that have a regulatory role, is working to meet the requirements of the proposed new Government Directive on Regulating and Smart Regulations. 4
We will continue to develop and apply innovative approaches to the regulation of health products and food to improve and sustain the timeliness and efficiency of the regulatory process to address the concerns of Canadians regarding safety, effectiveness and access. We will develop new regulatory approaches for radio-pharmaceuticals used for diagnosis and radiation therapy; for product-specific health claims for foods; for drug product licensing to support early access to promising drug therapies; and for a national approach to clinical trials. As well, a new external charging regime will be developed as part of a long term funding strategy to ensure adequate resources to sustain regulatory performance for human and veterinary drugs and other therapeutic products. We will begin to review regulations that require the pre-market safety assessment and authorization of foods and food products before they can be offered for sale.
As part of Health Canada's initiative to strengthen the safety system for therapeutic products, we will strengthen the oversight of clinical trials and investigational testing of medical devices conducted in Canada, access points for patients to new and innovative therapies. The trials and investigational testing provide the evidence of safety and efficacy required by the Regulations before a product may receive a general market authorization from Health Canada. The Department will increase capacity in 2006-2007 to allow the continued assessment of an increased number of applications within targeted time-frames; strengthen capacity to assess clinical trial and investigational testing of adverse reaction reports in order to detect, communicate and act on safety signals; and engage sponsors earlier in the clinical trials process. Moreover, through the safety initiative, Health Canada will update the national standards for blood and for cells, tissues and organs, while continuing to develop and implement an appropriate regulatory framework for these components. A program for compliance inspections of establishments will also be implemented.
Health Canada will work with the federal, provincial and territorial health and agriculture agencies involved in administering the national food safety system to better respond to current and emerging food safety issues. A major initiative will be Canada's Strategy for Safe Food. It engages federal, provincial and territorial governments, industry, academia and consumer groups to improve the overall management of the food safety system in Canada by developing a common vision and national priorities, and national public health outcomes, targets and indicators.
We will continue to lead development of a federal Biotechnology Stewardship Framework to encourage an integrated approach to managing the risks and benefits of biotechnology products and services in the public interest.
Information, education and outreach on health products, food and nutrition
Description: Responding to the growth of Canadian public interest in health issues by disseminating more information, written in language that is easy to understand, on how Canadians can maintain and improve their health.
| Expected Results | Performance Indicators |
|---|---|
|
Improved adoption in making safe and healthy choices for health products, food and nutrition |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 13.1 | 93.6 | 13.0 | 96.3 | 12.4 | 95.9 |
|
* Data is analysed and reported every two years. Health Canada will conduct a survey to assess the rate at which people use information to make health related choices. The target and actual rate will be determined in 2006-2007. The date to achieve the target is April 2008. *All financial figures in millions of dollars |
|||||
Health Canada will continue to provide useful information about the risks and benefits related to health products and food in order to help Canadians make informed decisions about their health. For example, we will develop food labelling policies as part of an integrated health and food safety policy tool kit that will be capable of responding more quickly and effectively to current and emerging health and food safety challenges. MedEffect, part of the initiative to strengthen the therapeutic products safety system, will enable us to maintain a website for posting safety alerts, public health advisories, press releases and notices for health professionals, consumers and other interested parties. 5 A similar website has been developed for veterinary drugs. 6 Also, as part of the overall effort to better inform Canadians, Health Canada will continue to provide balanced information on newer technologies and their applications, including biotechnology and nano-technology.
Promoting and supporting healthy eating and informing Canadians about risks related to the food supply are key in helping them to maintain and improve their health. For instance, Canada's Food Guide to Healthy Eating plays an important role in communicating healthy eating to Canadians. In 2006, a revised Food Guide will be completed and disseminated along with supporting materials, including a resource for intermediaries and a web-based interactive component. The Food Guide is being revised to ensure the guidance it offers continues to promote a pattern of eating that meets nutrient needs, promotes health, and minimizes the risk of nutrition-related chronic diseases. At the same time, the revision will strive to improve Canadians' understanding and application of the Food Guide.
Health Canada is working with the Public Health Agency of Canada to advance the healthy eating component of the Integrated Strategy on Healthy Living and Chronic Disease with a focus on multisectoral leadership and collaboration nationally and internationally. In addition, through this initiative Health Canada will develop nationally agreed upon nutrition indicators and targets, enhance efforts in knowledge development, synthesis and exchange, as well as develop and enhance nutrition surveillance capacity.
In 2006, an interactive Nutrition Label tool on the Health Canada website will be launched to help Canadians make informed choices about the foods they eat. 7 The tool will explain how the information on the new food label, which became mandatory on most prepackaged foods in December 2005, can be used to make healthy food choices. Enhancing awareness of nutrition labelling will also be accomplished through related initiatives such as the launch of the revised Canada's Food Guide.
Monitoring safety and therapeutic effectiveness and risk management
Description: Increasing the responsiveness to specific public health issues through the development of monitoring and surveillance systems and stronger compliance and enforcement activities.
| Expected Results | Performance Indicators |
|---|---|
|
Strengthened vigilance over safety and therapeutic effectiveness for health products and food on the market |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 104.8 | 1,171.3 | 103.7 | 1,205 | 99.2 | 1,200.3 |
|
* Health Canada's performance will be assessed through surveys, compliance rates and statistical analysis of adverse reaction data. Results from surveys such as those conducted by the Canadian Hemophilia Society will be reviewed as they are available. 8 Target of 95% compliance from inspections based on internal records has been set for health products. Implementation of new technologies will be used to meet internally harmonized standards for adverse reaction reports by 2007. *All financial figures in millions of dollars |
|||||
Recognizing the cross cutting nature of nutritionrelated surveillance activities, we will continue to transfer knowledge and build capacity needed for creating and improving evidence-based policies, programs and community interventions in collaboration with the following partners: Statistics Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, and stakeholder organizations such as the Federal/Provincial/Territorial Group on Nutrition, the Network on Healthy Eating and the Canadian Community Health Survey (CCHS) User's Group. Specific activities in 2006-2007 will include the approval of national nutrition indicators, the dissemination of a user's guide to assist nutrition stakeholders with the interpretation of the CCHS nutrition data, and the dissemination of a themed report on Food Security using CCHS data. 9
The 2006-2007 Canadian Health Measures Survey conducted by Statistics Canada will help increase Health Canada's capacity to monitor determinants of healthy eating. For example, the results of the survey will help decision-making related to the fortification of foods and the assessment of the prevalence of nutrition-related risk factors for cardiovascular disease and diabetes.
Health Canada will implement new safety measures to strengthen post-market surveillance activities designed to improve real world safety and effectiveness that are linked to the National Pharmaceutical Strategy. We have developed and used the Canadian Adverse Drug Reaction Information System to monitor suspected adverse reactions to health products. With significant advancements in technology and the establishment of international standards for data transmission, we will obtain and begin the implementation of a new and advanced adverse reaction monitoring system. The system will enable the collection and assessment of adverse reaction reports which span the entire life-cycle of health products, from pre-market testing to postmarket use, and will improve the overall efficiency of processing, managing and assessing adverse reaction reports. The system will comply with international standards recommended by the International Conference on Harmonization. For veterinary drugs, we have developed an adverse drug reaction reporting system and plan to develop a closer link between pre-market and post-market activities. In addition, Health Canada will consult with stakeholders and Environment Canada to develop environmental assessment regulations to help minimize the effects of therapeutic products on the environment.
Clinical trials require compliance inspections to protect people enrolled in them as well as the integrity and accuracy of the data that supports the application for new drugs bound for market. Through the initiative to strengthen the safety system, the number of clinical trial inspections in 2006-2007 will be increased to 60, equivalent to 1.5% of all clinical trials, with a view to achieving the international level of 2% in future years. This objective was recommended by the Standing Committee on Health in 2004. 10
Health Canada will continue to work with PHAC to implement the Canadian Pandemic Influenza Plan and to support the WHO's Global Agenda for Influenza Surveillance and Control. The Department will spend $6.2 million over 5 years, as well as $1.2 million ongoing, for planning and preparedness activities, providing regulatory guidance during the development of a prototype vaccine, developing a regulator's emergency implementation plan and designing an accelerated approval process.
Transparency, public accountability and stakeholder relationships
Description: Bringing more transparency to our decision-making processes by providing more accessible information about the science underpinning our decisions. Health Canada is also strengthening its capacity to involve the public in decisionmaking that in the past have been limited to scientific experts.
| Expected Results | Performance Indicators |
|---|---|
|
Improved public confidence and trust in the safety of health products, food and the regulatory system Level of public confidence of safety of health products, food, and nutrition |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 18.3 | 163.0 | 18.1 | 167.7 | 17.4 | 167.0 |
|
The progress of public confidence will be monitored with the aim of maintaining or increasing this level. The goal is to attain 85% of stakeholders holding a positive view on transparency and openness initiatives related to health products and food. Feedback from stakeholders will be sought as part of consultation activities to learn and improve over time. *All financial figures in millions of dollars |
|||||
Incorporating the views of citizens and stakeholders is critical for effective regulation in the public interest. Maintaining and strengthening public confidence is especially important in a world where the accelerating rate of scientific and technological advances carries both benefits and risks. Public confidence in the regulatory system, particularly as it applies to healthrelated products and services, is a prerequisite for ensuring that new and sustainable health innovations are available to and used by Canadians. As such, Health Canada is developing new tools to improve the transparency and openness of our regulatory system including convening national consultations and public forums on therapeutic health product and food safety issues important to Canadians, as well as developing and updating food safety guidelines, and assuring that new substances used in health products meet Canada's environmental assessment requirements.
Health Canada's Office of Paediatric Initiatives will serve as a focal point for an integrated approach to child health and safety issues across a number of regulatory areas, including food and nutrition and the safety and effectiveness of health products. The Office will bring together internal and external stakeholders to focus on these issues. The intended result for Canadians is improved, accessible information on the safety and effectiveness of health products used in children and on safe and nutritious food for them to consume.
In its April 2004 report, the Standing Committee on Health recommended that Canada introduce measures to ensure public confidence in the clinical trial process, starting with the disclosure of information about clinical trials through a public database. An External Working Group was formed to develop options for the registration and disclosure of clinical trial information. Further consultations will be held over the next year and will be informed by international efforts to create a harmonized approach to clinical trial registration and disclosure. 13 This process will allow for improved public access to meaningful clinical trial information while respecting the need for patient privacy and commercial confidentiality.
Endnotes
1 The Ayurvedic Medicine Information Session, Dietary Guidance Regional Consultations,
MedEffect Information Session, and Regional Stakeholder Food Forum.
2 www.pco-bcp.gc.ca/smartreg-regint/en/08/rpt_fnl.pdf
3 Numbers of Health Canada clinical trial applications and medical device investigational testing
applications
| 2001 | 2002 | 2003 | 2004 | 2005(Q1-2) | |
|---|---|---|---|---|---|
| Clinical Trial Applications (30-day) | 642 | 614 | 691 | 707 | 628 |
| Clinical Trial Applications (7-day) | 129 | 677 | 796 | 1,026 | 1,066 |
| Clinical Trial Application - Total | 771 | 1,291 | 1,487 | 1,733 | 1,694 |
| Investigational Testing | 89 | 94 | 100 | 123 | 131 |
4 www.cfr-crf.gc.ca
5 www.hc-sc.gc.ca/dhp-mps/medeff/index_e.html;
www.hc-sc.gc.ca/ahc-asc/branch-dirgen/hpfb-dgpsa/mhpd-dpsc/pediat_e.html
6 www.hc-sc.gc.ca/dhp-mps/vet/index_e.html
7 www.hc-sc.gc.ca/fn-an/surveill/index_e.html
www.hc-sc.gc.ca/fn-an/label-etiquet/nutrition/index_e.html
8 www.hemophilia.ca/en/10.1.4.php
9 www.hc-sc.gc.ca/fn-an/surveill/index_e.html
www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/index_e.html
10 www.hc-sc.gc.ca/dhp-mps/compli-conform/clini-pract-prat/report-rapport/2003-2004_tc-tm_e.html
11 www.oag-bvg.gc.ca/domino/reports.nsf/html/20040302ce.html
12 www.hc-sc.gc.ca/dhp-mps/compli-conform/info-prod/md-im/insp_strat_md-strat_insp_im_tc-tm_e.html
13 www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/proj/enreg-clini-info/index_e.html
Strategic Outcome: Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments
Program Activity - Healthy Environments & Consumer Safety
This program activity contributes to the Government of Canada Strategic Outcome: Healthy Canadians with Access to Quality Health Care.
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
|---|---|---|---|---|
| Gross expenditures | 289.9 | 305.4 | 301.9 | 302.3 |
| Less: Expected respendable revenues | 12.0 | 15.4 | 15.7 | 16.0 |
| Net expenditures | 277.9 | 289.9 | 286.2 | 286.3 |
| FTEs | 1,927 | 1,956 | 1,963 | 1,966 |
|
Notes: The increase in expenditures from 2005-2006 to 2006-2007 is mainly due to an increase in the level of funding for the Canadian Environmental Protection Act, which is partially offset by the Expenditure Review Committee (ERC) reduction. The decrease in expenditures from 2006-2007 to 2007-2008 is mainly due to a sunset of funding for Implementing the Border Air Quality Strategy and Related Air Quality Measures initiative. This decrease is partially offset by an increase in the level of funding for the Canadian Environmental Protection Act. Figures include an amount for other departmental and regional infrastructure costs supporting program delivery. |
||||
Program Activity Description
This program activity touches many elements of day-to-day living that have an impact on the health of Canadians. These include drinking water safety, air quality, radiation exposure, substance use and abuse (including alcohol), consumer product safety, tobacco and secondhand smoke, workplace health, and chemicals in the workplace and in the environment. We are also engaged in other health and safety related activities including the Government's public safety and anti-terrorism initiatives; chemical and nuclear emergency preparedness; inspection of food and potable water for the travelling public; and health contingency planning for visiting dignitaries. Much of this work is governed through legislation including the Food and Drugs Act, the Controlled Drugs and Substances Act, the Hazardous Products Act, the Radiation Emitting Devices Act, the Canadian Environmental Protection Act, the Tobacco Act, the Quarantine Act and others.
The Canadian public and governments are increasingly recognizing the critical link between human health, the physical environment and the economy. There is growing evidence that environmental factors, hazardous products, tobacco, alcohol and controlled substances contribute to a number of health problems including cancer, asthma and other illnesses and injuries which ultimately will have an impact on our health care system and society as a whole.
Building on our work to help protect the health of Canadians and in accordance with the principles of sustainable development, we will generate new research, partnerships and increased federal leadership to improve health outcomes, particularly for vulnerable populations such as children and young adults by:
- Reducing health and safety risks associated with tobacco consumption and the abuse of drugs, alcohol and other controlled substances; and
- Reducing risks to health and safety, and improving protection against harm associated with workplace and environmental hazards, consumer products (including cosmetics), radiation-emitting devices, new chemical substances and products of biotechnology.
From a health protection perspective, the Department will continue to focus on regulatory priorities such as Legislation Renewal and its impacts on the Hazardous Products Act, and we will also address regulations in the area of tobacco and other controlled substances. We will continue to improve national compliance and enforcement programs so they are effective, riskbased, and harmonized with provincial, territorial and international partners and stakeholders.
To protect the health of Canadians, we will continue to advance science and use evidence-based research to formulate our health promotion and harm prevention programs and policies. Health Canada will conduct research and use the science conducted by a network of external science organizations to respond to emerging health and safety challenges to Canadians. We will create a science plan which will outline the strategic scientific directions for our five key program areas for the coming years and we will continue to work closely with a number of other federal departments and agencies on common science-based issues, such as water.
We rely on professionals with expertise in applied sciences (e.g., toxicology, epidemiology, biology) and in both occupational and public health (e.g., nurses, medicine, psychologists, industrial hygienists) to achieve our key results for Canadians. The Healthy Environments and Consumer Safety Branch must compete with other organizations to attract highly qualified scientists and health professionals. To address this risk, we will develop and implement a Human Resources Strategy focussed on succession planning, learning, recruitment and retention in order to ensure we have the human resources to support our activities under this strategic outcome.
The broad mandate under this strategic outcome requires sustained partnerships that have a significant impact on the health of Canadians. For example, Health Canada is the lead on horizontal files that require significant interdepartmental collaboration, such as Canada's Drug Strategy. We contribute significantly to the Government of Canada's efforts on health and environment issues. For example, we share responsibility for the Canadian Environmental Protection Act with Environment Canada. We also work closely with Environment Canada on files such as climate change.
Internationally, we will continue to advance some of our key work with the U.S. on a range of issues such as children's health and the environment, sound risk-management of chemicals and the Canada-U.S. Memorandum of Understanding on Consumer Product Safety.
Within the Health Portfolio, the Healthy Environments and Consumer Safety Branch works in partnership with the Public Health Agency of Canada, First Nations and Inuit Health Branch, and Health Products and Food Branch to achieve integrated approaches to health. We also collaborate with the provinces and territories through various committees to develop guidelines on issues such as safe drinking water and to coordinate nuclear emergency preparedness actvities.
Performance Measurement Strategy
| Expected Results Branch PAA | Performance Indicators |
|---|---|
| Reduced risks to health and safety, and improved protection against harm associated with workplace and environmental hazards and consumer products (including cosmetics) |
|
| Reduced health and safety risks associated with tobacco consumption and the abuse of drugs, alcohol and other substances |
Prevalence of drug and substance abuse in Canada
|
Key Programs and Services
Tobacco Control
Description: As lead department for the Federal Tobacco Control Strategy, Health Canada supports the four pillars of prevention, cessation, protection and harm reduction. Health Canada works with partners to pilot and evaluate a range of stop smoking approaches. For instance, the Department develops and implements national and regional education campaigns that inform Canadians about the health impacts of smoking and that provide information and referrals to help more Canadians quit smoking, and reduce exposure to second hand smoke in public and private spaces. On the international front, Health Canada, through its International Affairs Directorate, supports tobacco control initiatives around the globe.
| Expected Results | Performance Indicators |
|---|---|
|
Reduce smoking prevalence among the Canadian population to 20%
Reduce number of cigarettes sold in Canada by 30% |
Smoking prevalence rate
Consumption rates - number of cigarettes sold in Canada
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 53.8 | 282 | 53.6 | 282 | 53.7 | 282 |
|
*All financial figures in millions of dollars |
|||||
Key Initiatives
As part of our commitment to monitor and report on the progress and success of the tobacco control initiative, Health Canada will conduct an evaluation of the first five years of the Federal Tobacco Control Strategy. In order to determine the direction for tobacco control over the next five years, the Department will analyse the outcomes from strategic planning sessions and consultations held with partners during the previous fiscal year. The knowledge derived from this evaluation will help us to most effectively focus Tobacco Control resources and activities for the future.
While considerable inroads have been made in reaching our targets and reducing smoking prevalence to 20% of the population, certain segments of the Canadian population continue to experience persistently high tobacco use, and further improvements to the smoking prevalence rate will depend upon addressing tobacco use among this population. Historically, young adults aged 20 to 24 have had the highest rates of smoking as compared with rates for all other age groups in the Canadian population, and this trend continues. For the first half of 2005, the smoking prevalence for those aged 20 to 24 was 27% as compared to 20% for the entire population 15 years of age and older (CTUMS). Therefore, the Department will focus its attention on youth and young adults who are most at risk of smoking, and will work with the provinces and territories to identify appropriate interventions and future directions for this important demographic. We will also provide support for targeted prevention and cessation activities of the no-smoking message through youth engagement initiatives, such as Health Canada's "Youth Action Committee on Tobacco", which will engage youth from across the country to help young people in their schools and communities live smoke-free.
Drug Strategy and Controlled Substances
Description: Health Canada administers the Controlled Drugs and Substances Act (CDSA) and its regulations, develops harm reduction and promotion strategies to combat alcohol and drug abuse (including health promotion activities targeted at youth), and provides expert scientific advice and drug analysis services to law enforcement agencies. Health Canada leads Canada's Drug Strategy, which was renewed in 2003. The Strategy is designed to coordinate and enhance substance abuse programs across the country, and strengthen knowledge and partnerships in the areas of prevention, treatment, harm reduction and enforcement to create healthier Canadians and communities.
Health Canada uses a variety of partnerships and funding vehicles to fulfill its mandate in this area. The Drug Strategy Community Initiatives Fund (DSCIF) was recently established to fund initiatives at the national, regional, provincial/territorial and local levels in two broad areas: health promotion and prevention, and harm reduction. DSCIF is delivered through Health Canada's national and regional offices and Northern Secretariat, with an overall aim to address problematic substance use and to promote public awareness of alcohol and other drug issues. The Alcohol and Drug Treatment and Rehabilitation Program (ADTR) aims to improve treatment for women and youth who are dealing with substance abuse problems. Health Canada also provides funding for the treatment component of Drug Treatment Courts.
| Expected Results | Performance Indicators |
|---|---|
|
Reduced health and safety risks associated with the abuse of drugs, alcohol and other controlled substances by managing the Controlled Drugs and Substances Act and its Regulations, and providing national leadership for Canada's Drug Strategy. |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 65.1 | 347 | 64.8 | 347 | 65.0 | 347 |
|
*All financial figures in millions of dollars |
|||||
Key Initiatives
Advancing the National Framework for Action to Reduce the Harms Associated with Alcohol and Other Drugs and Substances in Canada continues. Key planned components include the initiation of the Canadian Alcohol and Drug Use Monitoring Survey; the development of a National Alcohol Strategy; the implementation of the National Strategy to Combat the Production and Distribution of Marihuana and Synthetic Drugs and the Diversion of Precursor Chemicals; the development of a national and integrated approach to psychotropic pharmaceuticals; and the establishment of single website access to information about Canada's Drug Strategy. There will be an increase in research done in Canada on drug and alcohol abuse, and a detailed analysis of the Canadian Addiction Survey (CAS) of unique components (e.g., alcohol, youth, etc.) in order to support sound, evidence-based decision-making.
We will develop a strategy to enhance our inspection capacity for compliance with the Controlled Drugs and Substances Act (CDSA) and its regulations, in particular the Precursor Control Regulations. From a regional perspective, we will work on the provincial Alberta Methamphetamine Partnership Strategy Committee on Illicit Drug Use.
Marihuana is categorized as a controlled substance. The Marihuana Medical Access Regulations allow people who are suffering from grave and debilitating illnesses access to marihuana. It is important to note that the Regulations deal exclusively with the medical use of marihuana. Through authority of the Marihuana Medical Access Regulations, we will proceed with the development of a pilot project to assess the feasibility of distributing marihuana for medical purposes through the conventional pharmacy-based drug distribution system.
Safe Environments
Description: The environment continues to be a key determinant of health for all Canadians. Recent studies demonstrate that environmental factors contribute to a number of health problems. Air pollution, for example, is a factor in 5,900 deaths per year in Ontario and is responsible for 30% of asthma and 5% of cancers in children. Improving the health of Canadians by addressing environmental factors will strengthen their quality of life. The Safe Environments Programme promotes and protects the health of Canadians by identifying, assessing and managing health risks posed by environmental factors in living, working and recreational environments. The scope of activities encompassed within this area includes research on drinking water, air quality, contaminated sites, climate change, and vulnerable populations assessment of risks from environmental impacts, as well as preparedness for environmental emergencies. Health Canada is also the lead for coordinating Canada's preparedness for nuclear emergencies under the Federal Nuclear Emergency Plan.
| Expected Results | Performance Indicators |
|---|---|
|
Availability and Canada-wide adoption of measures to control the risks to human health posed by environmental contaminants Increased knowledge, understanding and involvement by Canadians in environmental health issues Science-based decision-making within Canada regarding health risks of environmental contaminants** Improved scientific knowledge and capacity within the Canadian scientific community and international collaboration on environmental health issues to ensure that Canadians have increased confidence in environmental health information and protection mechanisms* |
** Please Note: Work is in progress to develop a better set of indicators for these expected results by Fall 2006 |
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 68.4 | 576 | 68.1 | 582 | 68.3 | 584 |
|
*All financial figures in millions of dollars |
|||||
Key Initiatives
Health Canada's Sustainable Development Strategy (2004-2007) reflects our commitment to protect the health of Canadians from environmental threats. Under this strategy, the department will advance the development of the Guidelines for Canadian Drinking Water Quality, and an integrated source-to-tap approach to drinking water quality in Canada. In collaboration with other federal departments, Health Canada will develop a "Federal Waterborne Contamination and Illness Response Protocol" to ensure a coordinated and systematic federal approach to dealing with outbreaks of waterborne illness and contamination of drinking water. Health Canada is also partnering with all three levels of government on the Technical Advisory Committee on Safe Drinking Water (TACSDW) to effectively address public health issues pertaining to drinking water in Alberta.
To assist in monitoring health risks attributable to changes in air quality, the Department will continue to build on its recently completed collaborative international research to develop an Air Health Indicator and will release the Air Quality Benefits Assessment Tool to help quantify the health impacts of changes in air pollution. In particular, the Safe Environments Programme in Ontario and Region is working to support the Canada-United States Border Air Quality Strategy, and will examine the impacts of air pollution on the health of children and other vulnerable populations, such as pregnant women and diabetics, in the Great Lakes Basin region. Health Canada will also complete and disseminate the Canadian Climate Change and Health Vulnerability Assessment in 2007, which will assess climate change impacts on human health and well-being.
To protect the health of Canadians from environmental contaminants, Health Canada will make additional progress under the Canadian Environmental Protection Act, 1999 (CEPA) by completing the identification and prioritization ("categorization") of the 23,000 Existing Substances on the Domestic Substances List. The Domestic Substances List (DSL) is a compilation of about 23,000 substances used, imported or manufactured in Canada for commercial use.
In partnership with the Public Health Agency of Canada and working through the joint Emergency Preparedness Sub-Committee on Chemical Emergencies, we will develop a joint Health Portfolio response plan for chemical emergencies in 2006 - 2007. Health Canada is also working with its provincial counterparts to strengthen guidelines to protect the health of Canadians in the event of a nuclear emergency, and will be participating in international nuclear emergency exercises to assess the implications of implementing radiation contamination counter-measures.
Product Safety
Description: As part of our legislative mandate, Health Canada identifies, assesses, manages and communicates to Canadians the health and safety hazards and health risks associated with: consumer products; hazardous workplace materials; cosmetics; new chemical substances; products of biotechnology; radiation produced by radiation emitting devices; environmental noise; and solar UV radiation.
To carry out this mandate, we advance critical research, carry out needed risk assessments and develop risk management strategies to minimize the exposure of Canadians to toxic substances in consumer, commercial, personal care and pharmaceutical products. The Department annually carries out health risk assessments of approximately 800 new chemicals and products of biotechnology notified under the New Substances Notification Regulations of the Canadian Environmental Protection Act, 1999. When a significant risk is identified, control measures are imposed.We identify which of the Food and Drugs Act substances in Canadian commerce between 1987 and September 2001 require priority assessment, and work with stakeholders on the process for notification of these substances. The Department also carries out screening level health risk assessments on existing micro-organisms, and both environmental and health risk assessments on new Food and Drugs Act substances, including risk management when necessary.
Health Canada's National Office of Workplace Hazardous Materials Information System (WHMIS) provides leadership to its federal, provincial and territorial MOU partners regarding effective hazard communication of workplace chemicals, including the delivery of training for WHMIS inspectors across the country. In addition, the Department will continue its involvement and participation in standardization work to ensure continued improvement of radiation emitting equipment safety.
| Expected Results** | Performance Indicators |
|---|---|
|
Reduced risk of death and injury from exposure to hazardous products and substances associated with: Consumer products; cosmetics; workplace chemicals; new chemical substances; products of biotechnology; radiation-emitting devices; environmental noise; solar UV radiation. |
|
| 2006-2007 | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 25.6 | 334 | 25.5 | 334 | 25.5 | 334 |
|
*All financial figures in millions of dollars |
|||||
Key Initiatives
In support of the government's commitment to the health of children, we will proceed with the implementation of the Lead Risk Reduction Strategy (LRRS) for Consumer Products. These measures will protect the health of Canadians by reducing health risks related to lead exposure. Lead is hazardous to health and is particularly dangerous for infants and young children because they are more susceptible to its harmful effects. The LRRS proposes maximum lead content limits for four categories of consumer products with which children are likely to interact. Regulations for each category will be developed separately, moving first on product groups where the risk to children is greatest. We will also check for compliance rates for products already regulated for lead content. Performance will be based on the removal of such hazardous products from the Canadian marketplace and the level of public awareness of risks.
Other regulatory and educational activities will also support the commitment to the protection of children's health. For example, to protect the health of children and reduce strangulation hazards that are associated with flexible loops employed in the manufacture and use of window covering products (mini-blinds and curtains), regulations will be developed to require mandatory adherence by importers, advertisers and retailers to safety standards for these corded window covering products.
To effectively manage the continuing incidence of skin cancer, we need to ensure that children develop healthy behaviours with regards to their outdoor activities. The best time to influence or change behaviours is at the time the behaviour is being established. This is the fundamental driver for the Sun Awareness Project, a school-based outreach program to teach primary school children and teenagers of both the benefits of sun exposure and the risks associated with excessive sun exposure. The Sun Awareness Project involves learning elements and exercises integrated into the regular teaching schedule at both primary and secondary schools.
At the World Summit on Sustainable Development in 2002, Canada made a commitment to fully implement the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals by 2008. The GHS is viewed globally as the basis for the sound management of chemicals, and enhances the protection of human health and the environment by harmonizing chemical hazard classification and communication internationally. Building on stakeholder consultations on the GHS, Health Canada will make further progress toward carrying out the legislative and regulatory changes needed for full implementation by 2008.
In carrying out our responsibilities under the Canadian Environmental Protection Act, 1999, a priority will be placed on working with Environment Canada to develop regulatory amendments to the New Substances Notification Regulations (Organisms) that reflect changes in the regulatory, policy and science environment, such as the production of transgenic, chimeric and cloned animals (e.g., livestock). In addition, we will work to develop appropriate environmental regulations for substances in products that are regulated under the Food and Drugs Act.
Workplace Health & Public Safety
Description: The Workplace Health and Public Safety Programme (WHPSP) provides services to protect the health and safety of the federal public sector, the travelling public, dignitaries visiting Canada, and others. It also establishes and promotes national workplace health and safety policies.
Health Canada will continue to provide occupational health services to nearly 200,000 federal employees working in Canada and overseas for approximately 100 departments and agencies. Health Canada delivers Employee Assistance Program services on a cost recovery basis to approximately 143 Canadian public and para-public sector organizations. Through the International Health Bureau, Health Canada provides emergency health services to Internationally Protected Persons and their families while they are on official visits to Canada.
The health of those who travel within Canada is protected through voluntary inspection programs for passenger conveyances to address public health risks relating to food, water and sanitation. Potable water regulations provide some authorities for inspection and enforcement of water quality on conveyances. As well, under the Quarantine Act and in fulfilment of WHO International Health Regulations, WHPSP Environmental Health Officers are responsible for inspecting and assessing conveyances, goods and cargo, and ordering the detention, remediation, removal and destruction when necessary to protect against the transmission of communicable quarantinable diseases.
| Expected Results** | Performance Indicators |
|---|---|
|
Healthy Public Service Improved Public Health for the Travelling Public |
|
| 2006-2007* | 2007-2008 | 2008-2009 | |||
|---|---|---|---|---|---|
| $ | FTEs | $ | FTEs | $ | FTEs |
| 24.5 | 417 | 24.4 | 418 | 24.5 | 419 |
|
*All financial figures in millions of dollars |
|||||
Key Initiatives
In light of newly emerging health threats, Health Canada works with the Public Health Agency of Canada (PHAC), Public Security and Emergency Preparedness Canada, and other organizations to plan, prepare and implement physical and psychosocial emergency responses to national health emergencies such as pandemic influenza and terrorist or suspected terrorist attacks. Health Canada supports departments and agencies in their emergency preparedness and response activities through the provision of advice, guidance, training, health evaluations, prophylaxis and immunizations and will continue to support PHAC with environmental quarantine services. We will also continue to offer psycho-social services in support of federalized emergency responders and federal public employees who provide essential services during and immediately following critical incidents.
We will develop approaches to better identify and manage mental health and addictions in the workplace. Areas of activity will focus on: preventing and mitigating mental disorders and addictions among federal employees through mental health promotion, early identification and referral; a disability case management program; and the development and implementation of a comprehensive federal workplace health strategy.
Web Links
Tobacco
Canada's Drug Strategy
Marihuana Medical Access Regulations
Scheduling of a number of controlled substances and development of other proposed amendments to the Precursor Control Regulations
Controlled Substances and Precursor Chemicals
Reducing the supply of and demand for drugs through prevention, harm reduction, treatment and enforcement programming.
National Framework for Action on Substance Use and Abuse.
National Research Agenda
Drug Strategy Community Initiatives Fund
The Drug Strategy Community Initiatives Fund -- At a Glance
Canada's Drug Strategy Campaign publications
Canada's Drug Strategy Publications
Climate Change
Air
Water
Canadian Environmental Protection Act
Human Health and the Canadian Environmental Protection Act (CEPA): An Overview
Healthy Living Initiative
Protect Yourself Don't Delay Learn the Facts about UV Rays
Globally Harmonized System
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS)
Lead Risk Reduction Strategy
Workplace Health
Workplace Health and Public Safety Programme
* Other programs and services that contribute to this program activity total $52.5 million for 2006-2007
Program Activity - Pest Control Product Regulation
This program activity contributes to the Government of Canada Strategic Outcome: Healthy Canadians with Access to Quality Health Care.
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
|---|---|---|---|---|
| Gross expenditures | 60.5 | 58.6 | 58.2 | 54.1 |
| Less: Expected respendable revenues | 5.9 | 7.0 | 7.0 | 7.0 |
| Net expenditures | 54.6 | 51.6 | 51.2 | 47.1 |
| FTEs | 675 | 652 | 656 | 604 |
|
Notes: The decrease in expenditures from 2005-2006 to 2006-2007 is mainly due to the Expenditure Review Committee (ERC) reduction. The decrease in expenditures from 2006-2007 to 2008-2009 is mainly due to a decrease in the level of funding for Building Public and Stakeholder Confidence in Pesticide Regulation initiative. Figures include an amount for other departmental and regional infrastructure costs supporting program delivery. |
||||
Program Activity Description
Health Canada's program activity, Pest Control Product Regulation, protects human health and the environment by minimizing risks associated with pesticides imported, sold, or used in Canada. The activity is strengthened through the use of sound, progressive science, modern risk assessment, risk management approaches and innovative approaches to sustainable pest management.
Science is the foundation for Health Canada's activities related to Pest Control Product Regulations. We conduct assessments of risk to human health and the environment arising from exposure to chemical and biological pesticides as well as assessments of the value of these products. In support of this work, we develop assessment methodologies, pesticide testing protocols, risk reduction strategies and risk management tools. Scientific expertise is in place in the following areas: toxicology, environmental toxicology, analytical chemistry, environmental fate and chemistry, biochemistry, endocrinology, ecology, crop science, plant pathology, entomology, occupational and bystander assessment, and aggregate and cumulative assessment.
To meet the primary objective of this program activity "to protect the health of Canadians and the environment from unacceptable risks associated with pest control products", we collaborate with experts in a variety of disciplines throughout the Health Portfolio and with the five natural resource departments. We also work with: the Canadian Food Inspection Agency and provincial governments on compliance activities; with Agriculture and Agri-food Canada to develop risk reduction strategies and improve access to specialized pest control; and with a federal interdepartmental working group on pesticide research and monitoring. This working group provides the opportunity for us to discuss our research and monitoring needs, as well as obtain information on the levels of pesticides in the environment, effects on human health and the environment, testing protocols, risk reduction practices, pest control alternatives, and pesticides for minor use. It also gives our partners the opportunity to effectively link their research results to regulatory decisions, and, at the same time, it will improve our regulatory decision making process for pesticides.
Advisory groups play an important role in decisionmaking at Health Canada. The Minister's Pest Management Advisory Council allows for dialogue between stakeholders and Health Canada. The Economic Management Advisory Committee provides strategic advice on improving efficiency and cost effectiveness, and the Federal, Provincial, Territorial Committee on Pest Management and Pesticides helps strengthen federal, provincial and territorial relationships in the area of pest management and pesticides. The Committee also provides advice and direction to federal, provincial, and territorial governments on programs, policies and issues.
The major human resource challenge for this program activity is to keep up with the pace of growing scientific knowledge and industry innovation. We will need to continue to recruit additional resources that have the appropriate knowledge base, and develop and train staff in a number of scientific disciplines.
This program activity has three main objectives: protected health and environment; increased use of reduced risk pest management practices and products; and increased public and stakeholder confidence in pesticide regulation. To achieve these objectives we focus on five main activities that respond to a number of challenges facing Health Canada such as consumer demands, globalization, and rapid scientific and technological change. They are:
1. Regulatory Improvement
The new Pest Control Products Act, which came into force June 28, 2006, will strengthen health and environmental protection by enshrining in legislation modern risk assessment and risk management approaches that are currently applied as a policy matter. These include specific protection for infants and children, accounting for pesticide exposure from all sources (including food and water), and considering cumulative effects of pesticides that act in the same way. It will also continue to support pesticide risk reduction by ensuring that only pesticides that make a useful contribution to pest management are registered and by expediting the registration of lower-risk products. The registration system will be made more transparent by establishing a public registry to allow access to test data detailed evaluation reports on registered pesticides. Health Canada will continue to share scientific results on pesticides with provincial, territorial and international regulators to enhance the process for international joint reviews of pesticides.We will share sustainable pest management practices with provinces and territories to enhance agricultural stewardship. As a result, Canadian growers will have improved access to newer and safer pesticides so they can be more competitive in the marketplace.
In addition, the new PCPA will strengthen postregistration controls for all products. These requirements include: reporting by pesticide companies of adverse effects related to their pesticide products; re-evaluation of older chemicals on a cyclical basis; public transparency regarding the basis of regulatory decisions; and ensuring in legislation the special review program to address potential identified specific concerns for registered products.
To support the need to provide Canadians with a transparent and participatory regulatory process, we will transform our work from traditional business interfaces and information delivery practices to interactive, web-based practices. This transformation will allow information to be delivered, stored, accessed, retrieved, exchanged and used more effectively and efficiently, and will lead to more efficient and cost-effective management of the regulatory process.
2. New Pest Control Product Registration
New pesticides undergo an extensive pre-market assessment by Health Canada to ensure their use poses no unacceptable risks. This includes an assessment of human health risk (including worker and bystander exposure), food residues, environmental risk (including environmental fate and potential effects on wildlife), and an assessment of value. Assessments are carried out using the most modern scientific methods available and meet international best practices.
To provide for continual updating of our assessments, we are participating in a joint review program with the United States through NAFTA that is being expanded globally through the Organisation for Economic Co-operation and Development (OECD). This furthers the goals of timely registrations, harmonization and work sharing efforts to ensure pesticide risk assessments are efficient and benefit from the best science available internationally. Public consultation will continue to be used routinely for the development of major science policies and for registration decisions.
3. Registered Pest Control Product Evaluation
We re-evaluate older pesticides currently on the market to determine if their continued use is acceptable in consideration of modern data and current scientific approaches. Significant public consultation is undertaken on risk assessments and risk management proposals to engage stakeholders, including registrants, other government departments, growers and their associations, other non-governmental organizations, as well as the general public.
Risk mitigation measures will continue to be implemented where required to address concerns regarding risks that could emerge during the re-evaluation of a chemical. As required, under the PCPA, we will continue to work with the Environmental Protection Agency in the United States on a proposed approach to re-evaluation and develop a plan to work cooperatively on future re-evaluations.
4. National Pesticide Compliance Program
We have the ongoing responsibility to help protect the health of Canadians and their environment by facilitating, encouraging and maximizing compliance with the PCPA and its regulations. Where non-compliance is detected, we apply the appropriate enforcement (e.g., education, monetary penalties or prosecution). Health Canada promotes and monitors compliance with the Act and Regulations principally through its National Pesticide Compliance Program (NPCP).
The NPCP includes programs that address regional, multi-regional or national compliance and enforcement problems and issues. Much of this work is accomplished through a regional network of designated officials who inspect and investigate those who manufacture, distribute and use pesticides. An example of compliance activities is the monitoring of pesticide use in grape and blueberry production that will be done this coming year. Other pesticides will be monitored as issues arise.
In addition, we will continue to work in partnership with provincial and other federal regulators and will explore further opportunities for coordination and collaboration with international organizations. Specifically, in 2006-2007 Health Canada plans, through an OECD workshop, to continue to develop performance indicators for the compliance area.
5. Pesticide Risk Reduction in Agriculture
The Pesticide Risk Reduction Program supports the objectives of the new Pest Control Products Act to facilitate access to reduced risk products and enhance sustainability in agriculture. It is a growerled, commodity-based program that is jointly facilitated by the Sustainable Pest Management Section of the Pest Management Regulatory Agency and the Pest Management Centre of Agriculture and Agri-Food Canada (AAFC). The goal of the program is to improve the sustainability of Canadian agricultural commodities through the development and implementation of commodity-based risk reduction strategies. Benefits resulting from this program will include the development and adoption of alternative pest management practices through applied research into reduced risk alternative tools and biopesticides.
AAFC and Health Canada will continue working with stakeholders to develop commodity-specific pesticide strategies for twenty priority crops including apples, potatoes, dry beans and greenhouse vegetables. Active stakeholder participation in building and implementing strategies is critical to the success of the program.
Performance Measurement Strategy
| Expected Results | Performance Indicators |
|---|---|
|
Access to safer pesticides
Strengthened compliance with PCPA and Regulations Users informed of reduced risk practices Transparency of pesticide regulation Improved regulatory efficiencies and cost effectiveness
Informed public and stakeholders |
|
Web Links
Pest Management Regulatory Agency (PMRA)
home page: Health Canada's Pest Management Regulatory Agency (PMRA)
PMRA Strategic Plan 2003-2008
The Strategic Plan - Pest Management Regulatory Agency (PMRA)
Strategic Outcome: Better Health Outcomes and Reduction of Health Inequalities Between First Nations and Inuit and Other Canadians
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
|---|---|---|---|---|
| Gross expenditures | 1,930.8 | 2,124.6 | 2,139.7 | 2,159.2 |
| Less: Expected respendable revenues | 3.4 | 5.5 | 5.5 | 5.5 |
| Net expenditures | 1,927.5 | 2,119.1 | 2,134.2 | 2,153.7 |
| FTEs | 2,722 | 2,884 | 2,883 | 2,857 |
|
Notes: The increase in expenditures from 2005-2006 to 2006-2007 is mainly due to the yearly growth of the Indian Envelope and an increase in the funding level for the Follow-Up to the Special Meeting of First Ministers and Aboriginal Leaders (September 12, 2004). This increase is partially offset by the Expenditure Review Committee (ERC) reduction. The increase in the expenditures from 2006-2007 to 2007-2008 is mainly due to the yearly growth of the Indian Envelope and an increase in the funding level for the Follow-Up to the Special Meeting of First Ministers and Aboriginal Leaders (September 12, 2004). This increase is partially offset by the Expenditure Review Committee (ERC) reduction. The increase in expenditures from 2007-2008 to 2008-2009 is mainly due to the yearly growth of the Indian Envelope. This increase is partially offset by a decrease in funding for the sunset of the Implementation of the First Nation Water Management Systems initiative. The change in the FTEs is due to the increase of the salary component of the operating budget. Figures include an amount for other departmental and regional infrastructure costs supporting program delivery. |
||||
Program Activity Description
The objectives of Health Canada's First Nations and Inuit health program activity are improving health outcomes, ensuring the availability of and access to quality health services, and supporting greater control of the health system by First Nations and Inuit.
To achieve these goals, the Department must face many of the same challenges as other Canadian health care providers such as increasing costs, health human resource shortages and an aging population. The First Nations and Inuit health system has additional challenges due to rapidly growing populations with a higher than national average rate of injuries and disease burden, and a population living largely in remote and rural areas.
Within this context, Health Canada will focus on four key priority areas in 2006-2007: continuing to provide health-related programs and services; improving quality and access to health-related programs and services; promoting healthy living and disease prevention; and improving accountability and performance measurement. These priorities recognize the importance that determinants of health such as education and family income play in improving health outcomes, as well as the need for innovation in the field of health. They will also be informed by a government review of the health commitments of the 2005 Meeting of First Ministers and Aboriginal Leaders.
Continued health-related programs and services
Health Canada provides a range of First Nations and Inuit health programs and services that will continue into 2006-2007. In partnership with First Nations and Inuit, we will continue to provide primary health care services in approximately 200 remote communities by approximately 600 nurses through nursing stations and community health centres in remote and/or isolated communities. Through our regional offices, we also deliver programs focussed on children and youth, mental health and addictions, chronic diseases, environmental health, and communicable and noncommunicable disease prevention. These services supplement and support the services that provincial, territorial and regional health authorities provide.
The Non-Insured Health Benefits (NIHB) coverage of drugs, dental care, vision care, medical supplies and equipment, short-term crisis intervention mental health services, and medical transportation will continue to be available to all 765,000 registered Indians and recognized Inuit in Canada, regardless of residency.
Health Canada works closely with our health partners and other federal departments. We support the Public Health Agency of Canada in its delivery of Children and Youth programming through the Aboriginal Head Start in Urban and Northern Communities program as well as a number of pan-Aboriginal programs. We also work closely with Indian and Northern Affairs Canada through the First Nations Water Management Strategy to ensure that all First Nations communities across Canada have access to a safe and reliable water supply.
Improving quality of and access to health-related programs and services
The key elements of this priority include: working towards seamless integration of services; increasing the number of Aboriginal health professionals; support for accreditation; improved community dental capacity; and capital improvements and investments.
We will work to make progress towards better integration of federal, provincial and territorial health programming and services to First Nations and Inuit and to ensure that services meet the needs of Aboriginal peoples. This will include implementation of the Aboriginal Health Transition Fund, which was designed to enable federal, provincial and territorial governments, First Nations governments who deliver health care services, and Aboriginal communities to devise new ways to integrate and adapt existing health services. Attention will also be given to implementation of best practices and lessons learned from a series of Health Integration Initiative pilot projects.
Health Canada will continue to fund the Aboriginal Health Human Resources Initiative to increase the long-term supply of First Nations, Inuit and Métis health professionals. In order to focus on Aboriginal youth, scholarship and bursary funds will be made available to eligible youth who pursue post-secondary studies in health support. To meet the continuing challenge of recruitment and retention of nurses to support health services in communities, we will provide ongoing professional development and continuing education opportunities. In partnership with the Canadian Nurses Association, we will launch a National Nursing Portal to provide critical support to nurses in rural and remote areas.
Health Canada will also continue to support the development and implementation of First Nations and Inuit accreditation and quality improvement activities. This will increase the number of accredited health care services in First Nations communities and ensure that the health care provided is responsive to the needs of the communities. We will also continue to implement, in selected communities, the Children's Oral Health Initiative to improve the oral health of First Nations children. This will focus on increasing the awareness of preventive oral health care and positive self-care practices for parents and caregivers, and will serve to increase the capacity of communities to deliver and maintain dental public health initiatives.
Finally, Health Canada supports the construction, operation, maintenance and environmental management of on-reserve health facilities and staff residences. In 2006-2007, fifteen health facilities will be constructed or expanded, and recapitalization initiatives (repairs, replacements, upgrades) will improve the working environment of clients and staff, and enhance the quality of health care services offered at the community level. In 2006-2007, Health Canada will also invest $1.2 million in environmental remediation and assessment to ensure operations of health facilities in First Nations communities meet environmental codes and requirements and are consistent with the Department's commitments to sustainable development.
Promoting healthy living and disease prevention
This priority focuses on maternal and child health, mental wellness, suicide prevention, prevention of chronic disease, communicable disease readiness, and safe drinking water. Initiatives have been put in place, such as a Maternal and Child Health (MCH) program to further expand and enhance the continuum of services provided and to improve health and social outcomes for pregnant women and families with infants and young children within a targeted number of First Nations and Inuit communities. In 2006-2007, Health Canada will continue to expand the number of sites and spaces available for Aboriginal Head Start On Reserve (AHSOR) children and will provide training to AHSOR workers.
Health Canada will oversee the development of a strategic action plan in 2006-2007 to improve mental wellness outcomes for First Nations and Inuit. Among other issues, the strategic action plan will guide Health Canada's efforts to more effectively position its current programming so that they are better able to serve the diverse needs of Aboriginal communities.
We will also continue to implement the National Aboriginal Youth Suicide Prevention Strategy (NAYSPS). This new strategy will establish projects in a targeted number of Aboriginal communities, where it will focus on building a solid foundation for effective approaches for preventing youth suicide. The activities supported by NAYSPS will include: skills training; tool and resource development; and primary prevention and awareness initiatives that promote mental wellness and youth resiliency. The strategy will also develop protocols to respond to communities in crisis and support various research projects. In addition to these new activities, Health Canada will continue to offer a continuum of mental health and emotional support services to former students of residential schools and their families as Canada renews its efforts to resolve Indian Residential School legal claims more expeditiously in 2006-2007.
Health Canada will continue its efforts to address high rates of chronic disease within the Aboriginal community. In particular, the Department will enhance the Aboriginal Diabetes Initiative by increasing the level of community-based funding that communities can access to expand promotion, prevention and care activities. Beyond our efforts to address diabetes, we will develop a First Nations and Inuit Chronic Disease Prevention Strategic Plan that will be developed in partnership with key stakeholders and experts and will inform the development of future chronic disease prevention approaches.
In light of federal responsibilities to protect First Nations communities against health risks associated with communicable diseases, Health Canada will begin to implement Communicable Disease Emergencies Plans. Efforts will be focussed on increasing emergency planning and response capacity at the regional and community levels, strengthening collaborative relationships with provinces, territories and stakeholders and ensuring that emergency supplies are purchased and readily available to First Nations and Inuit communities. Health Canada will develop and pilot test Pandemic Influenza Plans in First Nations and Inuit communities by the end of 2006-2007.
Finally, through the Water Management Strategy, Health Canada will work in partnership with First Nations communities (except the Yukon and the Territories), to implement drinking water monitoring as per the Guidelines for Canadian Drinking Water Quality. This will involve the development of options and an action plan for the implementation of a regulatory regime for drinking water in First Nations communities. Health Canada will investigate potential drinking water problems and provide advice and recommendations to First Nations communities and federal partners such as Indian and Northern Affairs Canada. Health Canada is also actively involved in the development of community-based education and awareness activities on drinking water quality issues.
Improving accountability and performance measurement
Health Canada has developed a strategy to monitor, measure progress and report on program performance results. This includes establishing performance measurement strategies in consultation with the organizations delivering the services at the community level.
We will undertake efforts to improve health surveillance and information analysis, including data development, data analysis, research evidence to support priority-setting and decision-making on health-related investments. For example, as a further enhancement to the Aboriginal Diabetes Initiative, Health Canada will begin to support the development of an Aboriginal-specific diabetes research agenda and will increase the Department's surveillance activities in an effort to identify research priorities that will inform future diabetes programming.
Health Canada also draws information from evaluation and review studies on areas for improvement. In 2006-2007, we will conduct a joint evaluation with Indian and Northern Affairs Canada on the First Nations Water Management Strategy. The Department will also develop processes and tools and collect information for evaluating primary health care, immunization and mental health programs. It will finalize the evaluation of the Home and Community Care program.
Performance Measurement Strategy
The First Nations and Inuit Health program has established expected results and performance indicators to assess progress towards the achievement of the strategic outcome. Use of the information below will contribute to providing a snapshot of the health status of First Nations and Inuit.
| Expected Results | Performance Indicators |
|---|---|
| Strengthened community programs; better health protection; improved primary health care; and access to non-insured health benefits contribute to improved health status of First Nations and Inuit individuals, families and communities. |
|
Key Programs and Services
The following describes six key program areas that Health Canada will continue to be engaged in throughout 2006-2007: children and youth; mental health and addictions; chronic disease and injury prevention; environmental health and research; communicable disease control; and primary health care.
Children and Youth Programs
Description: These programs are designed to collectively improve the cultural, emotional, intellectual and physical growth and development of First Nations and Inuit infants, children and youth. Programs targeting maternal, infant and child health, increasing children's knowledge of language and culture, and increasing children's readiness for school are the main priorities of the Department's children and youth programming. These programs include: Aboriginal Head Start on Reserve; the Canada Prenatal Nutrition Program; the Fetal Alcohol Spectrum Disorder program; and the Maternal Child Health program.
| Expected Results | Performance Indicators |
|---|---|
|
Improved continuum of programs and supports in First Nations and Inuit communities Increased participation of First Nations and Inuit individuals, families, and communities in programs and supports |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 102.2 | 6.1 | 109.8 | 6.1 | 115.7 | 6.1 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Mental Health and Addictions Programs
Description: These programs provide culturally appropriate counseling services, addiction prevention and promotion services and mental wellness services that are largely delivered by Aboriginal people. These programs include: Building Healthy Communities; the Brighter Futures program; the National Native Alcohol and Drug Abuse Program (NNADAP) - Residential Treatment; the National Native Alcohol and Drug Abuse Program - Community based; the Youth Solvent Abuse Program; the First Nations and Inuit Tobacco Control Strategy; the National Aboriginal Youth Suicide Prevention Strategy; the Labrador Innu Comprehensive Healing Strategy; and the Indian Residential Schools-Mental Health Support Program.
| Expected Results | Performance Indicators |
|---|---|
|
Improved continuum of programs and services in First Nations and Inuit communities Increased participation of First Nations and Inuit individuals, families and communities in programs and services |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 139.1 | 5.8 | 130.7 | 4.3 | 133.3 | 4.4 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Chronic Disease and Injury Prevention Programs
Description: These programs support the development and implementation of community-based activities that promote healthy lifestyle choices and support healthy and active living. Over the long term, these programs will contribute to the prevention of chronic disease and injuries within First Nations and Inuit communities across Canada. These programs include: the Aboriginal Diabetes Initiative; Nutrition and Physical Activity Promotion; and Injury Prevention.
| Expected Results | Performance Indicators |
|---|---|
|
Improved continuum of programs and supports in First Nations and Inuit communities |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 34.9 | 2.8 | 45.1 | 2.8 | 50.1 | 2.8 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Environmental Health and Research Programs
Description: These programs are designed to reduce the risk of exposure to environmental health hazards by improving the capacity of communities to implement measures to manage, contain and control them. They also create and maintain healthy and safe community environments through: the investigation of potential environmental health-related outbreaks; raising awareness of environmental health hazards such as waterborne, foodborne and vector borne illnesses including health problems associated with poor indoor air quality, such as mould in housing. They provide for pest control and build community human resource capacity to adapt to environmental conditions, to maintain safe environments and to deal safely with environmental hazards. These programs include: First Nations Water Management Strategy; West Nile Virus; Contaminated Sites; Transportation of Dangerous Goods; Food Safety, Facilities Health Inspections; housing; and research.
| Expected Results | Performance Indicators |
|---|---|
|
Improved environmental health risk management |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 46.1 | 11.9 | 46.7 | 11.9 | 22.2 | 9.1 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Communicable Disease Control Programs
Description: These programs support public health needs and priorities in the design, implementation, management and delivery of programs to protect First Nations and Inuit communities from communicable diseases, and to implement measures to manage, contain and control risks of outbreak. These programs include: Tuberculosis; Immunization; HIV/AID; and Communicable Disease Emergencies.
| Expected Results | Performance Indicators |
|---|---|
|
Improved access to communicable disease prevention and control programs for First Nations and Inuit individuals, families, and communities |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 26.0 | 6.1 | 26.8 | 6.4 | 28.0 | 6.4 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Primary Health Care Programs
Description: Comprehensive health care services are provided to remote and/or isolated First Nations and Inuit settlements to supplement and support primary care services provided by provincial, territorial and/or regional health authorities. These include emergency and acute care health services. Health Canada ensures links to appropriate care by other health care providers and/or institutions as required by the client condition. The continuum of community health care and primary care services includes illness and injury prevention and health promotion activities. This includes the Home and Community Care Program and the Oral Health Strategy, for example.
| Expected Results | Performance Indicators |
|---|---|
|
Improved access to primary health care programs and services for First Nations and Inuit individuals, families and communities |
|
| 2006-2007* | 2007-2008* | 2008-2009* | |||
|---|---|---|---|---|---|
| $ | Salary $ | $ | Salary $ | $ | Salary $ |
| 232.9 | 66.7 | 233.7 | 66.4 | 237.8 | 65.7 |
|
* Based on the PAA with adjustments for approved Treasury Board submissions. It was assumed that no growth would be applied to new funding and non-envelope funding. It was also assumed that 3% growth would be applicable to envelope funding. Only salary dollars was provided because the information on the number of FTEs is not available at this level. ** All financial figures in millions of dollars |
|||||
Web Links
Other programs and services that contribute to this program activity total $1,539.9 million; for further information on those programs and services please see the following web links.
Aboriginal Head Start On Reserve
Aboriginal Head Start On Reserve
Fetal Alcohol Syndrome and Fetal Alcohol Effects
Fetal Alcohol Syndrome/Fetal Alcohol Effects
Aboriginal Diabetes Initiative
Injury Prevention
www.hc-sc.gc.ca/fnih-spni/promotion/injury-bless/index_e.html
Indian Residential Schools
National Native Alcohol and Drug Addictions Program
National Native Alcohol and Drug Abuse Program
Tobacco Control Strategy
NIHB
Non-Insured Health Benefits Directorate
Communicable Disease Control
Communicable Disease Control Division
Children's Oral Health Initiative
Children's Oral Health Initiative
Environmental Health
Drinking Water Quality
Home and Community Care
E-Health
Aboriginal Health Human Resource Initiative
Aboriginal Health Human Resources Initiative
Section 3: Supplementary Information
| ($ millions) | Forecast Spending 2005-2006 | Planned Spending 2006-2007 | Planned Spending 2007-2008 | Planned Spending 2008-2009 |
|---|---|---|---|---|
| Health Policy. Planning and Information | 448.4 | 288.6 | 218.2 | 214.9 |
| Health Products and Food | 262.4 | 303.2 | 298.4 | 283.1 |
| Healthy Environments and Consumer Safety | 287.8 | 306.1 | 301.7 | 301.3 |
| Pest Control Product Regulation | 58.3 | 58.7 | 58.2 | 54.1 |
| First Nations and Inuit Health | 1.867.7 | 2.087.9 | 2.126.9 | 2.138.8 |
| Budgetary Main Estimates (gross) | 2.924.6 | 3.044.5 | 3.003.4 | 2.992.2 |
| Less: Respendable Revenues | 68.9 | 69.1 | 69.4 | 69.7 |
| Total Main Estimates | 2.855.7 | 2.975.4 | 2.934.0 | 2.922.5 |
|
Adjustments: 1 Governor General Special Warrants: |
||||
| Operating Budget Carry Forward (horizontal item) | 14.1 | |||
| Additional funding in support of Aboriginal health further to the Special Meeting of First Ministers and Aboriginal Leaders on September 13. 2004 | 25.5 | |||
| Additional funding to territories for medical travel costs and health systems reform (TB vote 5) | 30.0 | |||
| Additional funding for initiatives related to the 10-Year Plan to Strengthen Health Care. such as wait times reduction. internationally educated health care professionals. and improved reporting to Canadians on the progress made in strengthening health care ($15.0 million from TB Vote 5) | 25.7 | |||
| Funding to ensure the safety of therapeutic products. including enhanced clinical trials oversight. monitoring of drugs and medical devices in the marketplace. and the implementation of new regulations for blood transfusion and organ transplantation (horizontal item) | 2.6 | |||
| Funding to enhance early learning and childcare programs for First Nations on reserve (horizontal item) | 6.1 | |||
| Additional funding for health risk assessments and protection measures related to the Canadian Environmental Protection Act | 1.7 | |||
| Activities to mitigate the impact of the Bovine Spongiform Encephalopathy (BSE) crisis (horizontal item) | 1.1 | |||
| Funding to deliver federal programs and services. including health in two Labrador Innu communities (Labrador Innu Comprehensive Healing Strategy) (horizontal item) | 3.9 | |||
| Funding to continue the Government's plan to establish core genomics research and development capacity (horizontal item) | 0.2 | |||
| Additional funding for the Access to Medicines Program which provides affordable access to Canadian patented pharmaceuticals for the treatment of HIV/AIDS. malaria. tuberculosis and other epidemics. in the least developed and developing countries | 0.7 | |||
| Funding related to the assessment. management and remediation of federal contaminated sites (horizontal item) | 0.6 | |||
| Funding to strengthen initiatives in support of the Canadian Strategy on HIV/AIDS in Canada (horizontal item) ($0.8 million from TB Vote 5) | 1.2 | |||
| Funding to improve the capacity to detect and the readiness to respond to a potential pandemic influenza outbreak including emergency preparedness. antiviral stockpiling and rapid vaccine development technology (horizontal item) | 0.4 | |||
| Funding for the environmental clean-up of the Sydney Tar Ponds and Coke Oven Sites in the Muggah Creek Watershed (horizontal item) | 0.1 | |||
| Funding to launch an integrated public health strategy to reduce the impact of chronic disease by promoting healthy living including specific initiatives to combat diabetes. cancer and cardiovascular disease (horizontal item) | 0.2 | |||
| Funding to undertake projects related to the development and application of biotechnology (Canadian Biotechnology Strategy) (horizontal item) | 0.2 | |||
| Funding for the development of and reporting on environmental indicators related to clean air. clean water and greenhouse gas emissions (horizontal item) | 0.2 | |||
| Funding for the delivery of federal programs and services. including health. to the O-Pipon-Na-Piwin Cree Nation (horizontal item) | 0.1 | 1.5 | 1.6 | 0.4 |
| Less: Spending authorities available | -61.0 | |||
| Other adjustments: | ||||
| Collective Agreements | 41.4 | |||
| Joint Career Transition Committee (TB Vote 10) | 0.1 | |||
| Adjustment - Statutory Items | 0.4 | |||
| EBP Adjustment | 7.9 | |||
| Funding for the Genomics Research and Development Initiative. under the auspices of the Canadian Biotechnology Strategy | 4.0 | |||
| Funding to Recognize a Landless Band and for the Registration of Newfoundland Indians | 7.3 | 7.5 | 7.8 | |
| Funding for the Winter Olympics | 0.6 | |||
| One year extension of funding authority for First Nations and Inuit Non-Insured Health Benefits Program Review | 30.0 | |||
| Funding for the Settlement Agreement for Indian Residential Schools | 2.0 | 11.0 | ||
| Funding for Avian and Pandemic Influenza Preparedness. with a Focus on Animal and Human Health | 1.5 | 4.0 | 4.0 | |
| Government Wide Efficiencies - Procurement Savings | -4.6 | |||
| Year End Lapse 2 | -66.9 | |||
| Total Adjustments | 36.3 | 35.7 | 15.1 | 27.8 |
| Total Planned Spending 3 | 2,892.0 | 3,011.1 | 2,949.1 | 2,950.3 |
| Less: Non-respendable Revenue | 8.9 | 8.9 | 8.9 | 8.9 |
| Plus: Cost of services received without charge 4 | 85.6 | 84.7 | 84.6 | 84.6 |
| Total Departmental Spending | 2,968.7 | 3,086.9 | 3,024.8 | 3,026.0 |
| Full-Time Equivalents 5 | 8,544.0 | 8,711.0 | 8,773.0 | 8,671.0 |
|
1 Adjustments reflect Governor General Special Warrants and TB Vote 5 Access for
2005-2006. |
||||
| Project Title | Estimated Completion Date1 |
|---|---|
| Recently Completed Internal Audits | |
| Review of the Administration of the Health Canada Contract with First Canadian Health Management Corporation Inc. | Approved by the DA&EC on April 7, 2005 |
| Follow-up of the Directed Audit of Société Santé en Français Inc. | Approved by the DA&EC on November 1, 2005 |
| Upcoming Internal Audits | |
| Audit of the Handling of Controlled Drug Substances (CDS) in FNIHB Health Facilities within Two Selected Regions | Approved by the DA&EC April 2006 |
| Audit of Primary Health Care Transition Fund (PHCTF) Contributions to the Ministry of Health and Long Term Care of the Province of Ontario | Fall 2006 |
| Audit of Selected Administrative Areas | Summer 2006 |
| Audit of Health Canada Initiatives for GOL | Approved by the DA&EC June 2006 |
| Audit of IT Security in Health Canada | Fall 2006 |
| Audit of the Implementation of Corrective Measures Ordered by the Public Service Commission | Fall 2006 |
| Selected Results-Based Management Accountability Frameworks | January 2007 |
| Audit of Mental Health and Addictions Programs | January 2007 |
| Audit of the Drug Strategy and Controlled Substances Programme | January 2007 |
| Recently Completed Evaluations | |
| Evaluation of the Memorandum of Understanding between the Assistant Deputy Ministers and Regional Directors General | Approved by the DA&EC November 2005 |
| Evaluation of the Health Canada Innovation Fund | Approved by the DA&EC November 2005 |
| Evaluation of the Canada Health Infostructure Partnership Program | Approved by the DA&EC November 2005 |
| Impact Evaluation of the Health Transition Fund | Approved by the DA&EC November 2005 |
| Formative Review of the Research Management and Dissemination Division | Approved by the DA&EC November 2005 |
| Health Transfer Policy - FNIHB | Approved by the DA&EC April 2006 |
| Brighter Futures and Building Healthy Communities Program - FNIHB | Approved by the DA&EC April 2006 |
| Non-Insured Health Benefits Pilot Projects - FNIHB | Approved by the DA&EC April 2006 |
| Primary Health Care Transition Fund - interim evaluation - Health Policy Branch (HPB) | Approved by the DA&EC April 2006 |
| Cost Recovery in the Pest Management Regulatory Agency | Approved by the DA&EC April 2006 |
| Health Care Strategies and Policy Grant and Contribution programs - Performance Measurement System Review - HPB | Approved by the DA&EC June 2006 |
| Upcoming Evaluations | |
| Review of Evaluation and Performance Measurement at Health Canada - CFOB | Fall 2006 |
| First Nations and Inuit Home and Community Care Program - FNIHB | Fall 2006 |
| Canada Prenatal Nutrition Program - FNIHB | Fall 2006 |
| Aboriginal Diabetes Initiative - FNIHB | Winter 2006-2007 |
| Augmenting Health Canada's Response to Bovine Spongiform Encephalopathy (BSE) - BSE I phase II of Health Canada's and the PHAC's response to BSE in the areas of Risk Assessment and Targeted Research - BSE II | Fall 2006 |
| Federal Drinking Water Compliance Program (HECS) | Winter 2006-2007 |
| Federal Tobacco Control Strategy | March 2007 |
| Contribution Program for Improving Access to Health Services for Official Languages Minority Communities - HPB | Winter 2006-2007 |
| Canadian Regulatory System for Biotechnology - HPFB | Fall 2006 |
| Therapeutics Access Strategy - HPFB | Fall 2006 |
| Natural Health Products Research Program - HPFB | Fall 2006 |
| Drug Strategy and Controlled Substances Program, Canada's Drug Strategy Renewed Year 2 - HECSB | Fall 2006 |
| Canadian Environmental Protection Act - HECSB | Fall 2006 |
| Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products - PMRA | Fall 2006 |
| Expenditure Review Reductions and the Impact on Health Canada - DAEC/DPMED | Winter 2006-2007 |
| Contracting for Professional and Special Services in Health Canada - DAEC/DPMED | Winter 2006-2007 |
| 1 The 'Estimated Completion Date' is the date the internal audit or evaluation report is expected to be tabled for approval by Health Canada's Departmental Audit and Evaluation Committee (DA&EC). | |
Section 4: Other Items of Interest
Health Canada's Regional Operations - An Overview
On January 6, 2006, the Deputy Minister of Health and the Associate Deputy Minster of Health announced the establishment of the Public Affairs, Consultation and Regions Branch. This new Branch incorporates the Communications, Marketing and Consultation Directorate; the external and internal ombudsman services; and the regions. The Branch affords Health Canada an opportunity to better integrate national and regional perspectives in all policies and strategies, communications and consultation functions, and is key to a commitment of transparency on the part of the Department.
Over the course of the last year, an initiative has been underway that aims to improve service to Canadians by strengthening the Department's programs and regional role, and enhancing communication and collaboration. As such, the role of the Regional Directors General has been enhanced in order to fully realize their integral role as Health Canada's senior representatives in the regions, responsible for the management of all of Health Canada's regional operations and personnel. The creation of the new Branch complements this ongoing work and will enable the Department to continue to evolve in the context of a changing environment. It also fulfills Health Canada's continuing commitment to ensure both greater coherence and a consistent presence for the Department nationally and in each region across the country.
In recognition of the unique program and service delivery challenges and opportunities among a diverse and often remote northern population, responsibility for all Health Canada activities in the Northwest Territories, Yukon and Nunavut is now overseen by the Northern Secretariat component of Regional Operations. This consolidation of responsibility for the North, under the Northern Secretariat, will provide a consistent Territorial lens for Health Canada's policy and program development, and greater coherence to Health Canada programs and services in northern communities. Further, in consideration of the needs and priorities identified within the community and among stakeholders, the Manitoba and Saskatchewan Region has been re-organized so that Manitoba and Saskatchewan each now assume status as an individual region. This will serve to enhance communications and consultations within the Department and with Health Canada's numerous stakeholders, partners and the public.
Health Canada's presence across the country will continue to be reflected through program and service delivery tailored to meet the varied needs of each of the geographic regions it serves. This includes the British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec and Atlantic regions, as well as the Northern Secretariat. Over one third of Health Canada's employees work in communities outside of the National Capital Region. This regional proximity to clients provides the Department with specialized knowledge and capacity to assist in furthering departmental goals and priorities. Health Canada's Regions offer local intelligence and a community connection which serves to bring regional perspective and coherence into the design and delivery of health protection and promotion programs as well as national policy development. Such citizen-centred engagement helps to ensure that program delivery in the regions is representative of and responsive to local issues and priorities, while respecting national program integrity and accountability.
The close ties between regional offices and their counterparts in other federal departments provide frequent opportunities for collaboration and partnership. Representation by Health Canada's Regions on regional Federal Councils serves to support the broader federal government agenda. In addition, it allows Health Canada to play a key role in reflecting regional views in the development of national cross-departmental policy and in the design of program delivery.
Health Canada's Regions foster and strengthen effective, long standing relationships and associations with provincial, territorial and municipal governments and key stakeholders. These relationships serve to advance horizontal collaboration and facilitate multipartnered and inter-governmental initiatives. They also improve the Department's understanding of challenges and opportunities which cross program boundaries as provincial and territorial approaches to managing the health care agenda evolve.
Strategic and targeted use of the Health Canada Innovation Fund continues to provide Health Canada's Regions with the ability to create and leverage partnership opportunities in response to local issues and concerns. Whether in partnership with academic institutions, non-governmental community based organizations or health research foundations, Health Canada Innovation Fund initiatives seek to identify and answer the specific needs and priorities within local groups and communities.
Providing Support to Departmental Strategic Outcomes and Corporate Objectives
Strengthened knowledge base to address health priorities:
- Manage intergovernmental affairs; and
- Foster communication, consultation and stakeholder engagement within the Regions.
Safe and effective health products and food and information for healthy choices:
- Conduct surveillance, enforcement and compliance activities for health-related products;
- Assist in ensuring safe and effective health products and food and information for healthy choices through regional contributions to national policies, programs, and regulations; and
- Engage in consultation to build stakeholder relations and provide information for making healthy choices.
Reduced health and environmental risks from products and substances, and safer living and working environments:
- Conduct inspection and surveillance activities as well as health promotion activities related to consumer products, tobacco, controlled drugs and substances, and the environment;
- Establish marketplace and user inspection programs as well as compliance and promotional activities for pesticides;
- Conduct risk assessments and evaluations and provide health advice to federal employees, provinces and municipalities related to chemical contaminates and exposure levels, drinking water standards, and work environments.
Better health outcomes and reduction of health inequalities between Aboriginals and other Canadians:
- Direct provision of Non-Insured Health Benefits to First Nations and Inuit clients;
- Delivery of community-based health promotion and disease prevention programs for First Nations and Inuit populations;
- Delivery of Home and Community Care Program and addictions treatment services for First Nations and Inuit populations;
- Development and delivery of health protection programs and services for First Nations and Inuit populations;
- Capacity building in the areas of health information management and analysis for First Nations and Inuit populations;
- Provision of management capacity support and capital investments in First Nations and Inuit communities; and
- Collaborate in emergency preparedness and response and pandemic planning.
Corporate Management - Leadership and infrastructure to support the Department's Regional Operations:
- Ensure sound stewardship of both the human and financial resources of the Department through effective and accountable management and administration of assets, human resources, information technology, policy, planning, security and business continuity services.
Supporting Health Canada's Programs and Services
Health Canada requires efficient and effective corporate services to ensure that it has the capacity and capability to undertake its main activities of promoting, protecting and improving the health of Canadians. The following examples demonstrate the Department's ongoing efforts and commitment to strengthen its corporate services and management practices
Our human resources planning process responds to the human resources risks and challenges we encounter in support of our business objectives. As well as consolidating and realigning IT resources and positioning the Department to align with the Government of Canada common services initiatives and generate savings, The Way Forward information technology project will ensure that service levels are maintained for all clients and that our IT infrastructure is sustainable in support of Health Canada's programs.
Health Canada was an early adopter of the Government's Management Accountability Framework (MAF) and will continue its efforts to not only promote the MAF throughout the Department but to implement management improvement initiatives to strengthen stewardship and accountability including the Financial Management Control Framework, the Contract Management Framework, the Contract Requisitions and Reporting System, the Asset Management Framework and the Departmental Real Property Management Framework. We will use the results of our MAF Assessment and other management or government priorities to determine areas requiring further attention or action. In addition, the Department will continue to share information and, as required, coordinate efforts with other Portfolio members.
We have also recently begun a department-wide operational planning process and we will continue our efforts to enhance and improve this process. Not only does operational planning link expected results to the allocation of resources, it will assist in identifying key priority pressures within the Department as well as reviewing opportunities for reallocation of resources from lower to higher priorities.
We will continue to implement an Integrated Risk Management (IRM) Framework and to update the Department's Corporate Risk Profile and Internal Scan on a regular basis. As well, we will continue to systematically manage risk in key functional areas and decision-making processes through such tools as the risk-based audit plan, the risk communications framework, risk-based disposition of records, an information technology enterprise approach predicated on risk management and a risk-based approached to human resources classification and staffing. We will also complete the development of an internal Departmental Business Continuity Plan in the Event of a Pandemic Influenza Outbreak that will allow us to maintain operations to the extent possible while protecting the health and safety of employees.
Initiatives are also underway to review, improve and update privacy policies and practices to ensure that personal information is protected within Health Canada. For example, a Departmental Information Management Awareness campaign is reinforced by more in-depth courses on both access to information and privacy offered to employees throughout Health Canada. We also conduct Privacy Impact Assessments on an on-going basis to identify and address privacy risks related to Departmental programs or services that handle personal information.
We foster an ethical culture that best embodies the core values of Health Canada and the Public Service through our Centre for Workplace Ethics. The Centre collaborates as appropriate with the services of Internal Ombudsman and Informal Conflict Management System (ICMS) to assist staff or teams with specific and pressing ethical challenges or conflict related issues. Regular reporting on trends by these three services provides the Deputy Minister with a department-wide perspective on progress as well as an early identification mechanism for areas of concern.
Details on Transfer Payments Programs
|
Name of Transfer Payment Program: Contributions to the Organization for the Advancement of Aboriginal People's Health (OAAPH) |
||||
|
Start Date: April 2005 |
End Date: March 2010 |
|||
|
Description: to support the Organization for the Advancement of Aboriginal People's Health |
||||
|
Strategic outcomes: Improved knowledge-based activities (including research) related to the health of Aboriginal people and communities |
||||
|
Expected Results : Continued empowerment of Aboriginal peoples through advancements in knowledge and sharing of knowledge on aboriginal health |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
5.0 |
5.0 |
5.0 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
5.0 |
5.0 |
5.0 |
|
Planned Audits and Evaluations: N/A |
||||
|
Name of Transfer Payment Program: Payments to Indian bands, associations or groups for the control and provision of health services |
||||
|
Start Date: 1989 |
End Date: 2006 |
|||
|
Description: T o increase responsibility and control by Indian communities of their own health care and to effect improvement in the health conditions of Indian people. |
||||
|
Strategic outcomes: Strengthened and enhanced accountability of community leaders to community members in transferred communities regarding the management and the delivery of health programs and services |
||||
|
Expected Results: Increased control or accountability by First Nations communities of health care services. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
209.6 |
217.9 |
227.9 |
233.9 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA First Nations and Inuit Health |
209.6 |
217.9 |
227.9 |
233.9 |
|
Planned *Audits and Evaluations: N/A |
||||
*Recipients are required to provide year end financial audited statements. Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Health Governance and Infrastructure Support (HG/IS) |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: Governance and Infrastructure Support to the First Nations and Inuit Health System |
||||
|
Strategic outcomes: Contributes to the improved health status of First Nations and Inuit individuals, families and communities through strengthened governance and infrastructure support. |
||||
|
Expected Results: Improved health status of FNI through strengthened governance and infrastructure |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
167.6 |
188.3 |
179.1 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
167.6 |
188.3 |
179.1 |
|
Planned *Audits and Evaluations: Evaluation
|
||||
* Contribution compliance audits are completed every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Community Programs |
||||
|
Start Date: April 1, 2005 |
End Date: March 2010 |
|||
|
Description: community programs support child and maternal-child health; mental health promotion; addictions prevention and treatment; chronic disease prevention and health promotion services. |
||||
|
Strategic outcomes: Contributes to the improved health status of First Nations and Inuit individuals, families and communities through strengthened community programs and supports. |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
211.3 |
228.6 |
243.5 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
211.3 |
228.6 |
243.5 |
|
Planned *Audits and Evaluations:
|
||||
* Recipients are required to provide year end financial audited statements. Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Health Facilities and Capital Program |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: Provides funding to eligible recipients for the construction acquisition, leasing, operation and maintenance of nursing stations, health centres, health stations, health offices, treatment centres, staff residences, and operational support buildings. |
||||
|
Strategic outcomes: Modern and well maintained health care facilities and residences that support effective health program delivery. |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
53.1 |
51.2 |
48.6 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
53.1 |
51.2 |
48.6 |
|
Planned *Audits and Evaluations: N/A |
||||
** Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Health Benefits |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: A limited range of medically necessary health-related goods and services which supplement those provided through other private or provincial/territorial health insurance plans is provided to registered Indians and recognized Inuit. Benefits include drugs, dental care, vision care, medical supplies and equipment, short-term crisis intervention mental health services, and transportation to access medical services not available on reserve or in the community of residence. |
||||
|
Strategic outcomes: Access to non-insured health benefits contributes to improved health status of First Nations and Inuit eligible clients |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
123.3 |
120.7 |
124.2 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
123.3 |
120.7 |
124.2 |
|
Planned *Audits and Evaluations: N/A |
||||
*Recipients are required to provide year end financial audited statements. Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Health Protection |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: Communicable Disease and Environmental Health and Research programs facilitate preparedness to implement measures in the control, management and containment of outbreaks of preventable diseases and improve management and control of environmental hazards. |
||||
|
Strategic outcomes: Health protection interventions contribute to improved health status of First Nations and Inuit individuals, families and communities. |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
9.7 |
10.3 |
9.2 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
9.7 |
10.3 |
9.2 |
|
||||
* Recipients are required to provide year end financial audited statements. Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for First Nations and Inuit Primary Health Care |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: Primary Health Care services include emergency and acute care health services, Community primary health care services which include illness and injury prevention and health promotion activities. These programs also include: the First Nations and Inuit Home and Community Care; and the Oral Health Strategy. |
||||
|
Strategic outcomes: Primary health care contributes to improved health status of First Nations and Inuit individuals, families and communities |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
119.7 |
121.2 |
123.0 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
119.7 |
121.2 |
123.0 |
|
Planned *Audits and Evaluations:
|
||||
* Recipients are required to provide year end financial audited statements. Contribution compliance audits are conducted every year for a sample of recipients.
|
Name of Transfer Payment Program: Contributions for Bigstone Non-Insured Health Benefits Pilot Project |
||||
|
Start Date: April, 2005 |
End Date: March 2010 |
|||
|
Description: Administration and delivery of benefits with Bigstone Health Commission to registered Indians and recognized Inuit. |
||||
|
Strategic outcomes: Access to non-insured health benefits contributes to improved health status of First Nations and Inuit eligible clients |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
||||
|
Total Contributions |
0 |
8.2 |
8.5 |
8.8 |
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
8.2 |
8.5 |
8.8 |
|
Planned Audits and Evaluations: 1 including a contribution audit |
||||
|
Name of Transfer Payment Program: Grant for Nunavut Medical Travel Fund |
||||
|
Start Date: April 2005 |
End Date: March 2010 |
|||
|
Description: To support the Nunavut medical travel fund |
||||
|
Strategic outcomes: To Improve Health System Sustainability in the Territories |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) First Nations and Inuit Health |
||||
|
Total Grants |
0 |
10.2 |
10.2 |
10.2 |
|
Total Contributions |
||||
|
Total Other Types of Transfer Payments |
||||
|
Program Activity (PA) First Nations and Inuit Health |
0 |
10.2 |
10.2 |
10.2 |
|
Planned Audits and Evaluations: N/A |
||||
|
Name of Transfer Payment Program: Named Grant to the Health Council of Canada |
||||
|
Start Date: September 1, 2004 |
Renewal Date: March 31, 2008 |
|||
|
Description: The mandate of the Health Council of Canada is to monitor and make annual public reports on the implementation of the 2003 First Ministers' Accord on Health Care Renewal and the 2004 Health Accord. |
||||
|
PAA Strategic Outcome #1: Strengthen knowledge base to address health and health care priorities. |
||||
|
Expected Results: Through monitoring and the annual public reporting on the progress achieved in implementing the 2003 First Ministers' Accord and the 2004 Health Accord, the Health Council of Canada will contribute to enhancing accountability and transparency in health system care reform. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) Health Policy, Planning and Information |
||||
|
Total Grants |
10.0 |
10.0 |
10.0 |
10.0 |
|
Total Contributions |
||||
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
10.0 |
10.0 |
10.0 |
10.0 |
|
Planned Audits and Evaluations: The Health Council of Canada publishes annual audited financial statements |
||||
|
Name of Transfer Payment Program: Grant to the Canadian Patient Safety Institute (CPSI) |
||||
|
Start Date: December 10, 2003 |
End Date: March 31, 2008 |
|||
|
Description: Establishment of a class grant program to support the federal government's interest in a federal/provincial/territorial partnership context, in achieving an accessible, high quality, sustainable and accountable health system adaptable to the needs of Canadians. |
||||
|
Strategic Outcome(s): Strengthen knowledge base to address health and health care priorities. To improve the quality of health care services by strengthening system coordination related to patient safety, including promoting national collaboration among key players |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) Health Policy, Planning and Information |
||||
|
Total Grants |
8.0 |
8.0 |
8.0 |
8.0 |
|
Total Contributions |
||||
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
8.0 |
8.0 |
8.0 |
8.0 |
| Planned Audits and Evaluations:The CPSI will undertake an initial program evaluation 3 years after the start date of the grant and once every 5 years thereafter. A full independent audit and auditor's report are required on an annual basis. | ||||
|
Name of Transfer Payment Program: Grant to the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) |
||||
|
Start Date: April 1, 2005 |
End Date: March 31, 2008 |
|||
|
Description: CCOHTA is an independent not-for-profit corporation established under the Canada Corporations Act , Part II, whose purpose is to co-ordinate, perform and facilitate the collection, analysis, creation and dissemination of information concerning the effectiveness and cost of technologies and drugs and their impact on health and the appropriateness of their use. The purpose of the Named Grant is to provide financial assistance to support CCOHTA's core business activities namely, Common Drug Review ("CDR"), Health Technology Assessment ("HTA") and Canadian Optimal Medication Prescribing and Utilization Service ("COMPUS"). |
||||
|
Strategic Outcome(s): Strengthen knowledge base to address health and health care priorities. The evidenced-based adoption, diffusion, management and utilization of health technologies within the Canadian health care system. |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) Health Policy, Planning and Information |
||||
|
Total Grants |
16.9 |
17.4 |
17.4 |
16.9 |
|
Total Contributions |
||||
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
16.9 |
17.4 |
17.4 |
16.9 |
|
Planned Audits and Evaluations: CCOHTA is to carry out and submit to the Minister, no later than June 30, 2007, an independent evaluation of CCOHTA's core business activities for the period of March 31 st , 2003 to March 31, 2007. |
||||
|
Name of Transfer Payment Program: Contributions for the Primary Health Care Transition Fund (PHCTF) |
||||
|
Start Date: June 13, 2001 |
End Date : September 30, 2006 |
|||
|
Description: $800 million PHCTF established in response to FMM 2000 commitment that improvements to primary health care are crucial to the renewal of the health care system overall. |
||||
|
Strategic Outcome(s): Strengthened knowledge base to address health and health care priorities Program Strategic outcomes: Effect quality improvement and cost-effectiveness of primary health care service delivery across Canada; knowledge development and translation initiatives to facilitate continuous improvement of primary health care service delivery; fund initiatives supporting primary health care renewal; support and coordinate the analysis and sharing of information on primary health care renewal; and, provide national leadership of primary health care service transformation in collaboration with provincial/territorial counterparts, stakeholder groups and other mechanisms. |
||||
|
Expected Results: Immediate Outcomes: 1) Acceleration of PHC renewal 2) Increased emphasis on PHC renewal 3) Increased collaboration on PHC renewal Intermediate Outcomes: 4) Improved infrastructure and systems to deliver PHC Intermediate Outcomes: 5) Enhanced knowledge and capacity to deliver PHC 6) A more integrated approach to the delivery of PHC Long-term Outcome: 7) Fundamental change in support of sustainable PHC systems * This outcome is not expected to happen within the life of the PHCTF; expected time frame is five to ten years. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) Health Policy, Planning and Information |
||||
|
Total Grants |
||||
|
Total Contributions |
248.9 |
75.6 |
0 |
0 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
248.9 |
75.6 |
0 |
0 |
|
Planned Audits and Evaluations The formative evaluation report has been completed and the PHCTF response and action plan to the report is expected to be approved by the end of fiscal year 05/06. The initiation of the summative evaluation of the PHCTF will also take place in fiscal year 05/06 with a completion in 06/07. Audits are currently being conducted on two initiative recipients funded by the PHCTF, these include the Bigstone Health Commission and the Northern and Aboriginal Population Health and Wellness Institute (NAPHWI). A management audit of the Ontario Per Capita file is also underway. All audits will be completed in fiscal year 05/06. |
||||
|
Name of Transfer Payment Program: Health Care Strategies and Policy Contribution Program |
||||
|
Start Date: September 24, 2002 |
End Date: March 31, 2007 |
|||
|
Description: To support the federal government's interests in achieving an accessible, high quality, sustainable and accountable health system adaptable to the needs of Canadians. |
||||
|
Strategic Outcome(s): Strengthened knowledge base to address health and health care priorities Increased evidence & knowledge base for decision-making in health care Increased collaboration/coordination on identified health care system issues/priorities |
||||
|
Expected Results: Reports, consultations, research and evaluation; educational models/tools and resources for health providers, health system managers and decision makers; innovative models for funding and delivery; innovative collaborations and/or coalitions; case studies and best practices; policy research documents; environmental scans, system and technology assessments; increased evidence and knowledge base for decision-making in health care; Performance Measurement System; evaluation framework; annual audits & ongoing monitoring. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) Health Policy, Planning and Information |
||||
|
Total Grants |
||||
|
Total Contributions |
21.0 |
29.1 |
38.4 |
36.6 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
21.0 |
29.1 |
38.4 |
36.6 |
|
Planned Audits and Evaluations: Overall Health Care Strategies, Policy Grant and Contribution Programs formative evaluation due on March 31, 2007. |
||||
|
Name of Transfer Payment Program: Contributions Program to improve access to health services for Official Language Minority Communities |
||||
|
Start Date: April 1, 2003 |
End Date: June 30, 2008 |
|||
|
Description: Contribution program to improve access to health services to official language minority communities. This initiative is part of the federal Action Plan for Official Languages. |
||||
|
Strategic outcomes: (a) improve access to health services for official language minority communities; (b) meet the needs and improve health services in official language minority communities, thereby enhancing the health of these communities; (c) improve the efficiency of the health system as a whole by improving health services for official language minority communities. These objectives are commensurate with Health Canada's mission to help people of Canada maintain and improve their health. More specifically, they relate to two of the departmental objectives namely ensuring high-quality health services that are efficient and accessible and reduce health inequalities in Canadian society. |
||||
|
Expected Results: Expected key program results have been developed with the Consultative Committees and are integrated into the Results-Based Management and Accountability Framework (RMAF) which have been developed for the Program. For both official language community there is two majors components: Component networking : the networking component is to mobilize the capacity of institutions, professionals and communities to foster the commitment to health services in both official languages for official language minority communities. Component training and retention of health professionnel : this component is to increase capacity for basic training, improve professional development and research, and promote the retention of health professionals in official language minority communities. |
||||
|
Networking Component A) French-speaking minority communities The expected results are to: -promote the establishment of strong, durable links among health sector stakeholders (health professionals, communities, policy makers, health care institutions, training institutions); -mitigate the geographic dispersal of Francophone and Anglophone minority communities and the isolation experienced by professionals; -promote communities ownership (in terms of the planning, development, strengthening or pursuit and promotion of improved access to health care in French); -maximize the use of existing resources and share best practices; -make health sector stakeholders more aware of the importance of language in health service delivery; -improve services to French-speaking minority communities by delivering high-quality health care and increasing their use; and - build capacity to provide health services in French through professional networking, and promote research capacity in French. |
||||
|
B) English-speaking minority communities The expected results are: -promote the establishment of strong, durable links among health sector stakeholders (health professionals, communities, policy makers, health facilities, training institutions); -mitigate the geographic dispersal of Anglophone minority communities and the isolation experienced by professionals; -promote communities ownership (in terms of the planning, development, strengthening or pursuit and promotion of improved access to health services in English); -maximize the use of existing resources and share best practices; - build capacity to provide health services in English through professional networking, and the participation of researchers in the minority language. |
||||
|
Training and Retention Of Health Professionnel A) Francophone minority communities The expected results are : -more Francophone professional health training through partnerships with the universities, colleges, etc.; -more incentives for recruitment and retention of health professionals able to serve Francophones, including greater motivation to remain in home regions after completing studies or professional training; -greater research capacity and better understanding of the needs of French-speaking minority communities; -more professionals to meet the needs of French-speaking minority communities; and greater satisfaction on the part of professionals and patients. |
||||
|
B) English-speaking minority communities The expected results are: -creation of mechanisms for promoting the professional training of health professionals to ensure that they can deliver health services to the Anglophone communities in the province; -improvement in the range and quality of services available to Anglophone minority communities in the Province of Quebec; -increase in the number of professionals to meet the needs of Anglophone minority communities; -a greater role for the Anglophone education sector in training and support for professionals who are working or intend to work in remote regions; and -greater satisfaction on the part of professionals and clients. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) |
||||
|
Total Grants |
||||
|
Total Contributions |
18.0 |
23.0 |
23.0 |
23.0 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
18.0 |
23.0 |
23.0 |
23.0 |
|
Planned Audits and Evaluations: The Program's Risk-based Audit Framework adopts an auditing approach, which assesses risk in proportion to the level of funds upon which contribution agreements are based. The Program will be audited by the Audit and Accountability Bureau Health Canada. The Program works in partnership among organizations created by official language communities themselves and Health Canada considers the risk associated with undesirable results to be low. The Department adopted monitoring practices and ongoing performance measures. The 2006 mid-term formative evaluation (on-going) will evaluate issues related to Program implementation and administration, including those that would apply to a partnership. The 2008 evaluation will evaluate the issues relating to Program relevance, attainment of results and cost-effectiveness ratio. |
||||
|
Name of Transfer Payment Program: Grant to the Canadian Blood Services (TB #826394) |
||||
|
Start Date : April 2000 |
End Date : Ongoing |
|||
|
Description : To support basic, applied and clinical research on blood safety and effectiveness issues through the auspices of Canadian blood services. |
||||
|
Strategic Outcomes : Access to Safe and Effective Health Products and Food and Information for Healthy Choices. |
||||
|
Expected Results : Continued improvements to basic applied and clinical research on blood safety and effectiveness. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) |
||||
|
Total Grants |
5.0 |
5.0 |
5.0 |
5.0 |
|
Total Contributions |
||||
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
5.0 |
5.0 |
5.0 |
5.0 |
|
Planned Audits and Evaluations: N/A |
||||
|
Name of Transfer Payment Program: Contributions in support of the Federal Tobacco Control Strategy (FTCS) |
||||
|
Start Date: 2001-2002 |
End Date: 2006-2007 |
|||
|
Description: The purpose of the Federal Tobacco Control Strategy (FTCS) Contribution Program is to contribute to the achievement of FTCS objectives through assistance to provinces or other bodies. In doing this, the Program supports the implementation of the four components of the Federal Tobacco Control Strategy, namely: Protection (to reduce access to tobacco and to regulate the product); Prevention (to reduce the uptake of tobacco and to create barriers to smoking); Cessation (to increase the number of quitters and reduce barriers to quitting); and Harm Reduction (to reduce harm to smokers and those exposed to tobacco). Contributions are provided to support the provinces and territories as well as key national and regional non-governmental organizations and others in order to build ongoing capacity for delivering effective tobacco prevention and cessation programs. Contributions are also used to advance tobacco control initiatives to reduce harm to smokers and those exposed to tobacco. In addition, as part of the FTCS Mass Media program, contributions are also used to fund regionally-based mass media initiatives in support of the four components. Contributions are required as Health Canada's efforts need to be coordinated and integrated with the efforts of its partners to achieve a sustained reduction in tobacco use. In addition, many of Health Canada's partners are in a better position, because of their particular expertise, to deliver certain FTCS activities or can deliver them in a more cost-effective manner. |
||||
|
Strategic outcomes: Reduces the health and safety risks associated with tobacco consumption by: regulating tobacco; developing and implementing initiatives to reduce or prevent the harm associated with tobacco use. The goal of the Federal Tobacco Control Strategy is to:
|
||||
|
Expected Results: The goal of the Federal Tobacco Control Strategy is to:
|
||||
|
Forecast Spending 2005-2006 * |
Planned Spending 2006-2007** |
Planned Spending 2007-2008*** |
Planned Spending 2008-2009*** |
|
|
Program Activity (PA) |
27.1 |
31.4 |
0 |
0 |
|
Total Grants |
||||
|
Total Contributions |
19.2 |
15.8 |
15.8 |
15.8 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
46.3 |
47.5 |
15.8 |
15.8 |
|
Planned Audits and Evaluations: The Federal Tobacco Control Strategy (FTCS) is currently being evaluated at its five-year mark. The evaluation is a full summative evaluation. It will examine the overall success of the strategy, cost-effectiveness, continued relevance as well as a review of management practices. The evaluation will be completed in the summer of 2006. |
||||
* based on P9 programme forecast
**one year extension using Minister's authority - not yet approved
*** no authorities - Treasury Board Submission yet to be submitted
|
Name of Transfer Payment Program: Alcohol and Drug Treatment and Rehabilitation (ADTR) Contribution Program. |
||||
|
Start Date: April 1, 1997 |
End Date: A-Base |
|||
|
Description: A cost-sharing program to provide payments to provinces and territories to support access to alcohol and drug treatment and rehabilitation programs. |
||||
|
Strategic outcomes: To assist in ensuring access for Canadians to effective alcohol and drug treatment and rehabilitation programs and services. Increased access to services by women and youth. Changes to provision of services for women and youth. Discussions began in 2004-2005 with the provinces and territories regarding the implementation of a performance measurement strategy. |
||||
|
Expected Results: Increased access to and utilization of alcohol and drug treatment and rehabilitation services by women and youth. |
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) |
||||
|
Total Grants |
||||
|
Total Contributions |
14.0 |
14.0 |
14.0 |
14.0 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
14.0 |
14.0 |
14.0 |
14.0 |
|
Planned Audits and Evaluations: The Drug Strategy and Controlled Substances Programme (DSCSP) anticipates completing its review of literature and varying data sources in building and supporting emerging evidence on the continued relevancy of the target population, women and youth by end of June 2006. |
||||
|
Name of Transfer Payment Program: Drug Strategy Community Initiatives Fund (DSCIF) |
||||
|
Start Date: April 2004 |
End Date: A-Base |
|||
|
Description: A contributions funding program under Canada's Drug Strategy to support community-based initiatives at the national, regional, provincial/territorial and local levels in two broad areas: health promotion and prevention, and harm reduction. It is delivered through Health Canada's national and regional offices and Northern Secretariat. |
||||
|
Strategic outcomes: The overall aim of the DSCIF is to address problematic substance use and to promote public awareness of alcohol and other drug issues |
||||
|
Expected Results:
|
||||
|
Forecast Spending 2005-2006 |
Planned Spending 2006-2007 |
Planned Spending 2007-2008 |
Planned Spending 2008-2009 |
|
|
Program Activity (PA) |
||||
|
Total Grants |
||||
|
Total Contributions |
10.8 |
9.9 |
10.8 |
10.5 |
|
Total Other Types of Transfer Payments |
||||
|
Total PA |
10.8 |
9.9 |
10.8 |
10.5 |
|
Planned Audits and Evaluations: Audit and evaluation activities regarding the Drug Strategy Community Initiatives Fund are reflected in the overall audit and evaluation plans of Canada's Drug Strategy (CDS). As such, DSCIF is a key component of the Interim Year Two Risk-Based Evaluation that is currently underway, and the Interim Year Five Outcome-Based Evaluation to take place in 2007-2008. |
||||
Conditional Grants (Foundations) for Health Canada
| Name of Recipient: Canada Health Infoway Inc. (Infoway) | ||
| Start Date: March 9, 2001 | End Date: not applicable | Total Funding: 1.2 Billion |
|
Description: Canada Health Infoway is a federally-funded independent not-for-profit organization with a mandate to foster and accelerate the development and adoption of electronic health information systems with compatible standards and communications technologies on a pan-Canadian basis. It is widely accepted that health information and communications technologies such as electronic health records (EHRs), telehealth and public health surveillance systems will significantly improve access to health care services, patient safety, quality of care and productivity. A recent study estimated that potential savings from a Canada-wide electronic health record similar to Infoway's model would be $6.1 billion annually. Infoway collaborates with the federal, provincial and territorial governments towards a common goal of modernizing Canada's health information systems. This collaborative approach reduces overall costs by coordinating efforts, avoiding duplication, taking advantage of economies of scale, replicating successful initiatives across the country, and sharing best practices. For example, Infoway's EHR Blueprint Architecture is being adopted across Canada by jurisdictions and vendors, saving time, effort, and dollars, and helping to ensure all systems will be interoperable; as well, some jurisdictions have saved both time and money by acquiring vendor solutions together, rather than individually. While Infoway provides advice and some funding to them, the provinces and territories are responsible for the actual system development, implementation and overall funding, including on-going operational costs. Electronic Health Record - Infoway's goal, endorsed by all jurisdictions, is to put in place the basic elements - i.e. patient and health professional registries; drug, laboratory and diagnotic imaging systems - of an interoperable EHR for 50% of Canadians by the end of 2009. Over 140 projects are completed or underway across Canada and it is expected that an active pace will continue to be maintained in 2006/07. The following provides examples of such projects: the Registries program comprises 10 completed and 14 active projects in 10 of 13 jurisdictions; approximately 30% of physicians have now been uniquely identified, a prerequisite for EHR use; with respect to Diagnostic Imaging, there are 4 completed and 9 active projects in 7 jurisdictions; the British Columbia Fraser Health Authority plans to have all of its twelve hospitals connected to its shared diagnostic imaging system by the end of 2005/06; in Southwestern Ontario, The Thames Valley Hospital Planning and Partnership is making significant headway as seven of its eight hospitals are now able to share diagnostic images; late in the summer of 2005, the Alberta government and Infoway announced a $189 million initiative to allow hospitals and clinics throughout Alberta to electronically capture and share patient X-rays and CT and MRI scans. Telehealth - Infoway is investing in projects to expand and sustain telehealth initiatives, particularly in rural and remote communities, including Aboriginal and official language minority communities. It is also working on linkages between telehealth and the EHR and increasing the integration of telemedicine activities into mainstream healthcare service delivery. Telehealth strategic plans have been put in place with most jurisdictions and telehealth solutions will be implemented in all jurisdictions by December 31, 2009. Health Surveillance Systems - Infoway conducted a comprehensive needs assessment and developed an implementation strategy. Based on this, they will invest in the implementation of a pan-Canadian health surveillance system focussed on infectious diseases, in particular on case, outbreak, immunization, and alerts management, building upon surveillance systems already in place in Canada. A national steering committee, made up of experts in both information technologies and public health representing all FPT jurisdictions, has completed the solution planning work and three streams of work are underway: solution procurement and integration, to be completed end of February 2006; public health surveillance standards review and development; and jurisdiction implementation planning sessions. |
||
| Strategic Outcome(s): Strengthened knowledge base to address health and health care priorities. | ||
|
Summary of Annual Plans of Recipient: In 2005/06, Infoway updated three-year joint technology and investment plans with the jurisdictions, providing a consolidated long-term 'road map' that aligns national and provincial e-health strategies. This national three-year plan will be updated annually by Infoway and the Deputy Ministers, and will serve to more tightly align the joint investments made by Infoway with the provinces and territories. As of March 31, 2006, it is expected that Infoway will have approved approximately $650 million in project investments, with additional downstream commitments of approximately $420 million. |
||
|
Planned Audit(s) and Evaluation(s): In 2005/06, independent third parties completed a financial audit, a compliance audit, and an evaluation to measure performance against outcomes set out in the Infoway Funding Agreement. In addition, Infoway prepared a Progress Report on surveillance systems. In 2006/07, independent third party financial and compliance audits will be undertaken. Independent performance evaluations are scheduled every five years. Infoway initiatives must generate value - measurable benefits - for the patients, providers and health care system. Therefore, Infoway's EHR solutions will be evaluated in the field to determine benefits, as well as utilization and satisfaction levels. The benefits evaluation framework is being enhanced and primary research will be stimulated in this area. |
||
| URL to Recipient site: Canada Health Infoway | ||
| Name of Foundation: Canadian Institute for Health Information (CIHI) | ||
|
Start Date: |
End Date: Roadmap I March 31, 2003 Roadmap II March 31, 2007 Roadmap II+ March 31, 2007 Roadmap III, March 31, 2010 |
Total Funding: Roadmap I $95M Roadmap II $95M Roadmap II+ $70M Roadmap III $110M |
|
Description: The Roadmap Initiative provides the financial support for the Canadian Institute for Health Information to:
|
||
|
Strategic outcomes: CIHI plays a major role in supporting the reporting commitments of various First Ministers Meetings and the First Ministers Accords on Health Care Reform. The key areas identified for action under the original Health Information Roadmap were:
|
||
|
Summary of Annual Plans: The 2005-06 Operational Plan and Budget was presented for approval to the Board of Directors at the June 2005 meeting, and then submitted to the Minister of Health. Some of the key projects include:
|
||
| Planned audit(s) and evaluation (s): CIHI will be conducted a complete third-party evaluation within one year of the completion of the project (i.e., 2008). | ||
| URL to Foundation site: The Canadian Institute for Health Information (CIHI) | ||
| Name of Recipient: Canadian Health Services Research Foundation | ||
| Start Date: 1996-97 | End Date: | Total Funding: approx $151.5 M |
|
Description: Total federal funding for the CHSRF is as follows (CHSRF's programs also receive funding from other sources):
|
||
|
Strategic Outcome(s): The NRF will support research personnel, research dissemination, and research projects on nursing management, organization, and policy at a level of $2.5 million per year for 10 years. The aim is to create high quality new knowledge; increase knowledge exchange between researchers and decision makers; and increase the capacity for evidencebased decisions. EXTRA aims to equip health service professionals and their organizations with the skills to find, assess, interpret and use research to better manage the Canadian health care system. CHSRF will continue to enrol 24 senior fellows annually in the two-year EXTRA training program designed to achieve: knowledge of research evidence; capacity to draw on system thinking; development of collaborative professional relationships; and the ability to introduce and manage evidence-based change. CHSRF's work contributes to Health Canada's aim of strengthening the knowledge base to address health and health care priorities. CHSRF's programs further the development of health human resources, provide health managers with tools to improve primary and continuing care, and support research on nursing issues from a health system perspective. |
||
|
Summary of Annual Plans of Recipient: CHSRF will continue its efforts on its four strategic objectives:
CHSRF will move to consolidate and add value to its research funding activity; this will include positioning more activities in relation to its four "flagship" programs:
Emphasis will be placed on creative knowledge transfer and providing increased support to decision makers, as well as on organizational excellence. CHSRF's partnership work is expected to change due to the increasing number of national healthrelated knowledge agencies and the drive to identify opportunities and common objectives; and the provision of more direct assistance to grant and award applicants to help acquire matching co-sponsorship funding. |
||
| Planned Audit and Evaluation: Financial statements are audited annually. CHSRF commissioned an International Review Panel Report in 2002 and is currently preparing the background work and the process for the 2nd international review in 2007. As part of the preparations for this international review in 2007, CHSRF created a comprehensive logic model in 2004/05 for its overall impact on evidence-based decision-making in the health sector. This model will guide the overall evaluation and has already been used, in adapted form, by other organizations in Canada to guide their activities and evaluations in the area of evidence-based decision-making in health systems. A compliance audit of funded research projects was conducted in 1999 and another compliance audit will be conducted in 2006. The foundation also commissioned an internal controls review in April 2005 with the implementation of the recommendations occurring in 2005 and 2006. Finally, the foundation drafted an enterprise risk management framework in 2005 with the final version being completed in 2006. | ||
| URL to Recipient site: The Canadian Health Services Research Foundation | ||
Horizontal Initiatives
| Horizontal Initiative: Canada's Drug Strategy | Lead Department(s): Health Canada | |||
| Start Date: CDS started in 1987; CDS Renewed 2003 - 2004* | End Date: Ongoing | Total Funding Allocated: $752.2M* | ||
|
Description: Canada's Drug Strategy (CDS) was first introduced in 1987 to address substance use and abuse issues in Canada through coordinated activities by various federal departments, governments and non - governmental organizations. In 1992, following some initial successes in the areas of prevention and treatment, Phase II was launched with an emphasis on Driving While Impaired. During Phase II of the CDS, changing government priorities resulted in less than half of the funding being applied to the Strategy making it difficult to fully address complex issues related to both supply and demand reduction. Under Canada's Drug Strategy Renewed (approved by Cabinet in May 2003), the CDS will continue to be a comprehensive inter - Departmental federal initiative designed to coordinate and enhance substance abuse programs, knowledge and partnerships in the areas of prevention, treatment, harm reduction and enforcement. For more information, please refer to Canada's Drug Strategy. |
||||
|
Shared Outcome(s): Improved Leadership - Setting directions and creating environments that support local action through community - based initiatives integrally linked to national objectives and targets Enhanced knowledge generation and management - Providing strengthened capacity to improve evidence - based policy and decision making by promoting leading - edge research, statistical monitoring of drug trends and evaluation of program effectiveness Enhanced partnerships and interventions - Discouraging substance abuse, targeting illegal conduct that threatens the safety and security of Canadians, and assisting those at risk from the effects of drugs by supporting partnerships and programs that focus on prevention, harm reduction, treatment and enforcement activities Improved modernization of relevant legislation and drug policies - Ensuring that legal and policy approaches underpinning CDS are coherent with and support the Strategy, by reviewing legislation and regulations for responsiveness to current requirements |
||||
|
Governance Structure(s): Health Canada (HC) An Assistant Deputy Minister Interdepartmental Steering Committee exists and is chaired by Health Canada. Working groups focussing on Communications, Research and Surveillance, Evaluation and Risk Management, and Emerging Issues have been established to support decision-making by the ADM Steering Committee and Health Canada provides secretariat to support these structures. In addition, small coordination units will be implemented in core federal departments and Health Canada's regional offices. Public Health Agency of Canada (PHAC) Department of Public Safety and Emergency Preparedness Canada (PSEPC) Royal Canadian Mounted Police (RCMP) Correctional Services Canada (CSC) Canada Border Services Agency (CBSA) Department of Justice(DOJ) Department of Foreign Affairs Canada (FAC) |
||||
| * CDS was initiated in 1987 and has undergone a number of reiterations in the past 17 years. CDS Renewed was approved in May of 2003. The financials presented reflect a start date of May 2003 and an end date of the 2004-2005 fiscal year. The funding allocation during this two year period is inclusive of both the enhanced funding received under CDS Renewed and a-base funding pertaining to activities undertaken in the area of demand and supply reduction. | ||||
| Federal Partners Involved in each program | Names of Programs | Total Allocation | Planned Spending for 2006-2007 | Expected Results for 2006-2007 |
|---|---|---|---|---|
|
1. Health Canada RCMP |
Promotion / Prevention & Public and Professional Education / Training Programs / Activities |
$4.87M $8.27M
$2.8M $15.94M |
$5.85M $5.26M
$11.11M |
Increased awareness of the nature, extent and consequences of substance use/abuse within the school, workplace and Aboriginal communities and among youth, professionals and the general public Improved skills/competencies in the delivery of programs |
|
2. Health Canada Correctional Services Canada (CSC) Department of Justice |
Treatment and Rehabilitation Programs / Activities |
$173.68M $31.60M
$2.60M $207.88M |
$87.58M $19.10M
$3.80M $110.48M |
Enhanced access and motivation to participate in treatment for substance abuse Reduction in risk behaviours/decisions and overall assessed substance abuse treatment needs |
|
3. Health Canada PHAC CSC
|
Research and Surveillance Programs / Activities |
$20.38M $2.90M $1.50M
$4.10M $28.88M |
$11.04M $ 1.0M $ 2.0M
$11.11M |
Increased knowledge and understanding of emerging trends and related consequences in the area of substance abuse and what works in preventing and treating substance use/abuse More evidence-based responses to substance use/abuse |
|
4. Health Canada Foreign Affairs Canada PSEPC |
Grants & Contributions Program |
$4.20M $5.00M $.31M
$9.51M |
$15.75M $2.90M $.10M
$18.75M |
Increased capacity/ability to identify, understand and address issues pertaining to the demand for and supply of illicit drugs and harmful substances |
|
5. Health Canada PSEPC |
Coordination and Collaboration Programs / Activities |
$6.42M $1.25M $9.51M |
$2.96M $.63M $3.59M |
Increased collaboration/ involvement of stakeholders Improved coordination/direction of efforts among CDS partners/stakeholders Enhanced credibility/influence of CDS in setting directions/policies in the area of supply and demand reduction |
| 6. Health Canada | Policy and Legislative Review and Development Programs / Activities | $2.6M | $1.55M | Improved policy and regulatory responses to the demand for and the supply of illicit drugs and harmful substances |
|
7. Health Canada RCMP |
Enforcement Programs / Activities |
$27.22M $150.17M $163.80M $12.10M $126.44M $479.73M |
$14.26M $79.07M $81.90M $ 5.90M $64.50M $235.63M |
Improved understanding and knowledge of drugs, related trends, and production and diversion methods Enhanced ability to detect and respond to the supply of illicit drugs and harmful substances |
| Total | $752.21M | $396.15M | ||
| Contact: Colleen Ryan, Manager, CDS Evaluation, Risk Management and Reporting, (613) 957-2867, colleen_ryan@hc-sc.gc.ca | ||||
| Approved by: Beth Pieterson | Date Approved: Beth Pieterson | |||
| Horizontal Initiative: Federal Tobacco Control Strategy | Lead Department(s): Health Canada | |||
| Start Date: 2001/02 | End Date: 2005/06 & ongoing | Total Funding Allocated: $560M (See note about Health Canada total funding in section 11) | ||
|
Description: The Federal Tobacco Control Strategy (FCTS) establishes a framework for a comprehensive, fully integrated, and multi-faceted approach to tobacco control. The FTCS is the federal contribution to the national tobacco control plan endorsed in 1999 by all Ministers of Health. It focuses on four mutually reinforcing components: protection, prevention, cessation and harm reduction, supplemented by effective use of public education campaigns to reach all Canadians. |
||||
|
Shared Outcome(s): The FTCS has five 10-year objectives (2001-2011):
Explore how to mandate changes to tobacco products to reduce health hazards. |
||||
|
Governance Structure(s): Resources for the implementation of the FTCS were allocated to a number of departments and agencies. Health Canada (HC) is the lead department in the FTCS and is responsible for regulating the manufacture, sale, labelling and promotion of tobacco products as well as developing, implementing and promoting initiatives that reduce or prevent the negative health impacts associated with smoking. The partner departments and agencies are:
|
||||
| Federal Partners Involved in each program | Names of Programs | Total Allocation | Planned Spending for 2006-2007 | Expected Results for 2006-2007 |
| 1. HC | FTCS |
$482.5M (Note: this original allocation has been affected by several cuts since the FTCS began. The reductions went towards funding other departmental and government priorities, i.e. $47M to CEPA, $32.5M held back as part of the Government Advertising Plan, and $3M, starting in 05/06, reallocated as part the Expenditure Review exercise.) |
$58.7M (TCP: $49M) (FNIHB: $9.7M) |
|
| 2. PSEPC | FTCS | $3.2M | $45,000 | Enhanced Partnership Arrangement with Akwesasne Mohawk Police |
| 3. Justice | FTCS | $10.0M | $1,326,445 |
|
| 4. RCMP | FTCS | $10.5M | $1,500,000 |
|
| 5. CRA | FTCS | $53.8M (total allotment to the former CCRA) | ||
| 6. CBSA | FTCS | (see row above) | $5.1M |
|
| * Note that Québecs statistics are not included in these figures which would be substantially higher. This province was not yet integrated into the Department of Justice's Icase system when the March 2005 statistics were consolidated. As of June/05, Québec has been integrated in Icase. | ||||
| Horizontal Initiative: Building Public Confidence in Lead Department(s): Pest Management Regulatory Pesticide Regulation and Improving Access to Agency (PMRA)--Health Canada (HC) Pest ManagementProducts | Lead Department(s): Pest Management Regulatory Agency (PMRA)--Health Canada (HC) | |||
| Start Date: 2002-2003 | End Date: 2008-2009 | Total Funding Allocated: | ||
|
Description: The initiative is a part of the federal government's commitments as outlined in the Treasury Board submission Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products. The Treasury Board submission and its associated Results-based Management and Accountability Framework (RMAF) describe the integrated approach by which initiatives will be measured, managed and reported throughout their life cycle. An important element of the commitments made through the Treasury Board submission is that stakeholders and public will be kept informed through a transparent management system. The participating departments will work together for shared outcomes; measure performance on delivery; and review progress achieved. This initiative incorporates efforts of six federal government partners to increase public and stakeholder confidence in the pesticide regulatory system, to protect health and environment, and to increase the competitiveness of the agri-food and forestry sectors. Research and monitoring in the area of pesticides is being coordinated with their regulation. Under this initiative, the presence and effects of pesticides in the environment, in marine and freshwater ecosystems, and in the forest environment are being monitored. The initiative enhances monitoring and enforcement of pesticide residue limits in foods, in feed, of pesticide residues in fertilizers, and pesticide guarantee verification for fertilizer-pesticide combinations. Reduced-risk pesticides and biological pesticides for forestry are being developed and their use facilitated. Commoditybased risk reduction strategies for the agriculture and agri-food sector are being developed and implemented. Programs improving access to agricultural minor-use pesticides and reduced-risk pesticides for agricultural use are being established. Research to support the introduction of minor-use pesticides that pose a reduced risk to the environment is being conducted. A reporting system to track adverse effects of pesticides has been developed, and information on these effects will be collected and recorded. Collectively, this work is being conducted to achieve public confidence in increased conservation and protection of human health and the environment while contributing to the competitiveness of Canada's agricultural sector. The information presented in this table has been organized along the following three main themes of this initiative:
|
||||
|
Shared Outcomes: Immediate Outcomes:
Intermediate Outcomes:
Final Outcome: Increased public and stakeholder confidence in pesticide regulation, protected health and environment as well as increased competitiveness of the agri-food and forestry sectors |
||||
|
Governance Structures: PMRA (HC)--Executive Director EC--Director General, Conservation Strategies Directorate and Director General, National Programs Directorate DFO--Director General, Fisheries, Environment and Biodiversity Science NRCan--Director General, Science Branch, Canadian Forest Service AAFC--Assistant Deputy Minister of the Farm Financial Programs Branch and Assistant Deputy Minister of Research Branch, Executive Director, Pest Management Centre CFIA--Vice President, Programs Deputy Minister Committee--Deputy Minister from Health and AAFC AAFC/PMRA Joint Management Committee: Assistant Deputy Minister of the Farm Financial Programs Branch, AAFC, Assistant Deputy Minister of Research Branch, AAFC, Executive Director, PMRA, Health Canada, Treasury Board Secretariat (ex-officio member) |
||||
| Federal Partners Involved in each program | Names of Programs | Total Allocation | Planned Spending for 2006-2007 | Expected Results for 2006-2007 |
|---|---|---|---|---|
|
AAFC, CFIA, DFO, EC, HC (PMRA), NRCan 1. AAFC |
I. Research and Monitoring (a) Conducting research to support the introduction of minor-use pesticides that pose a reduced risk to the environment. |
$8.0M |
$2.0M |
Following evaluation of research projects, continued funding for some as appropriate. Final reports and next steps for implementation of research results underway for projects completed in March, 2006. Initiation (in April, 2006)of approximately 20 new projects in Minor Use Research and Biopesticide Initiatives as a result of the November, 2005 Project Call. Results of 1 year of research work on these projects to be reported upon (April, 2007) Research planning, coordination continues with MOU Research Working Group |
| 2. CFIA | (b) Enhanced monitoring and enforcement of pesticide residue limits in food and feed. | $0.25M |
Identify food commodities consumed by targeted subgroup (children). Lab testing of an approximate 1500 samples per year. Follow-up inspections for non-compliant test sample results. Publish annual report of the findings of the National Chemical Residues Monitoring Program (NCRMP). Food recalls, as required, for risk mitigation and removal of hazardous foods from marketplace. |
|
| 2. CFIA | (c) Enhanced monitoring and enforcement of pesticide residues in fertilizers and pesticide guarantee verification in fertilizer-pesticide combinations. | $2.15M | $0.25M |
Develop monitoring and surveillance policies and processes to guide and advise operational staff on fertilizer-pesticide combinations and pesticide contaminated fertilizers. Increase interaction with the PMRA to obtain the most up-to-date pesticide safety and labelling information. Update the Compendium of Fertilizer-Use Pesticides, which contains information regarding registration, guarantees and proper labelling. Work to develop regulatory changes to facilitate updating of the Compendium more regularly, and, if successful, provide Compendium updates more regularly to the producers of mixtures and to the CFIA's inspection staff. Advise CFIA Operations on appropriate follow-up procedures and recommendations regarding the significance of sample analytical results. Sample fertilizer-pesticide combinations to verify guarantees. Sample fertilizers suspected to be contaminated with pesticides. Verify fertilizer-pesticide labels. Conduct investigation and compliance activities (anticipated based on sampling and inspection frequencies). Analyze samples submitted by inspectors. |
| 3. DFO | (d) Monitor and research the presence and effects of pesticides in marine and freshwater ecosystems. | $7.9M | $1.0M |
DFO will provide the PMRA with final reports on four regional National Fund projects:
DFO will provide the PMRA with a status report from DFO's Centre for Environmental Research on Pesticides (CERP). CERP will conduct studies to quantify impacts of exposure to pesticide residues in 2 model systems in Canada; one representative of prairie land use and another indicative of southern Ontario land use pattern. Impacts will be quantified in terms of reproductive success of the native fish populations as well as overall population numbers. After consultation with the PMRA, DFO will design and initiate new research projects related to the theme "Population Level Impacts of Pesticides on Fisheries Resources". Contribute to the Formative Evaluation of the Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products Horizontal Initiative. |
| 4. EC | (e) Monitor and research on presence and effects of pesticides in the environment. | $7.0M | $1.0M |
EC will:
EC Leads in specific research and monitoring themes have provided EC's Pesticide Program Coordinating Committee with a document highlighting each of their theme's results for the first three year cycle of work Themes include air and water surveillance; fish, amphibian and multitrophic aquatic effects; and, plant, mammal and bird terrestrial effects. Following three years of research we will obtain answers to questions regarding knowledge generation with highlights of findings, contribution to the initial Pesticide Science Fund (PSF) objectives (e.g., national in scope and linked to regulatory decision-making priority, advanced knowledge of pesticide fate and effects, etc.), contribution to future departmental priorities, links within EC and to other interdepartmental research/monitoring activities, leverage of complementary work and building of partnerships, scientific (or other) publications procuded and finally the theme's top five priorities for PSF (incl. research, monitoring, methods development, risk assessment and modelling). These documents were used by the Committee to prioritize research and monitoring activities for a second cycle of work beginning fiscal year 2006-07. Environmental priorities will be set according to the fundamentals of detecting change, understanding why it is changing, better understanding of what we can do about it, and using this information to inform decision makers and Canadians. Collected knowledge will be used in the context of EC's Competitiveness and Environmental Sustainability Framework (CESF) and applied to pesticides. This will support decisions related to national competitiveness, to the protection of the health and safety of Canadians as well as to the conservation of ecosystem functions. In order to better integrate and coordinate EC research with regulation, EC will continue to work with the PMRA in the implementation of the EC/PMRA MOU. The MOU has four components, Science Policy, Knowledge Generation, Issue Management and Compliance Promotion and Enforcement include many initiatives and activities not listed herein. EC will continue working on providing leadership in the development and implementation of a federal, co-ordinated pesticides science strategy for research and monitoring. As well EC will continue to contribute to PMRA's pesticide assessments where appropriate and will continue to provide science/policy advice on key Government of Canada policies as they relate to pesticide management and use in Canada. |
| 5. HC (PMRA) | (f) Linking pesticide regulation and research. | $4.2M | $0.8M |
Identify PMRA's research and monitoring priorities annually and communicate to 5NR partners through regular meetings and other avenues as needed. Facilitate discussion among the 5NR on identifying actions to address specific priorities, including collaborative research. Discuss with the 5NR how the results of their research and monitoring are used in regulatory decisions to build better linkages between research and regulation. Facilitate the two-way communication and coordination between regulation and research between governments within Canada (through PMRA's FPT Committee) and internationally as well as with the private and academic sectors, through presentations linking research and regulation at regional, national and international meetings.(e.g., through SETAC, CSA, IUPAC). To strengthen the framework in linking pesticide research and monitoring, develop a MOU amongst the 5NR on linking research to regulation. Improve risk assessment procedures particularly in the area of environmental fate prediction (e.g., water modelling and exposure assessment). Continue to improve and expand the use of probabilistic risk assessments. |
| 5. HC (PMRA) | (g) Conducting research to support the introduction of minor-use pesticides that pose a reduced risk to the environment. | $3.5M | $0.9M |
Advance the risk assessment methodologies through:
Facilitate access to reduced risk products, specifically low risk products, through developing, and publishing for external comment, guidance on registration of low risk products. Continue to develop a database on environmental toxicology and fate to guide decisions, internally and externally, on comparative risk and reduced risk products. Finalize and publish a Best Management Practices guide to reduce spray drift by applicators. Publish for public comment a document identifying various options to better communicate buffer zones on labels to applicators. |
| 6. NRCan | (h) Research and monitor pesticides in the forest environment. | $3.5M | $0.4M |
Third and final year of research work for four projects will be completed and results reported in final reports and publications. Provide results to clients/stakeholders and PMRA. The research projects are:
Refine research priorities and plan for request for new proposals, January 2007. |
|
AAFC HC (PMRA) 1. AAFC |
II. Developing and implementing commodity specific risk reduction strategies (a) Commodity based risk reduction strategies. |
$19.3 |
$2.5 |
Process to engage stakeholders in crop prioritization based on risk and needs assessments developed. Next wave of about 10 crop profiles to be finalized and published. Develop up to 5 risk reduction strategies and support implementation of priority projects as established with Technical Working Group/stakeholders. Fund research and implementation projects from November 2005 call for proposals. Follow-up from workshop on barriers to grower adoption of IPM practices Analysis of data from pilot pesticide use survey. Continued implementation of AAFC/PMRA joint communication plan. |
| 2. HC (PMRA) | (a) Commodity based risk reduction strategies (RR). | $25.7M |
$4.0M (2.0 for commodity strategies / 2.0 for RR product review) |
Planned staffing actions in 2006-2007, indeterminate positions. Ongoing consultations with stakeholders, Work share with other government departments and 5NRs. Work on pesticide risk indicator: consult, build and validate database. Refine, together with AAFC, prioritization criteria for determining priority crops for the program. Workshare with AAFC on crop profiles. Risk reduction strategies have been developed for pulse crops and canola. A long term fireblight management strategy has been developed for apples. Steering committee and working groups have been meeting to develop solutions to the identified priorities and implement steps to resolve these issues. Substantial progress has been made in developing strategies and forming steering committees to lead the strategies for a number of other crops, particularly, greenhouse vegetables, grape, peach, potato, strawberry and apple. Pursue risk reduction program for honey, Richardson ground squirrel and develop a work plan for forestry uses and needs. Consolidate and integrate all information collected with this program into the registration stream of the PMRA. Continue review of reduced-risk pesticides submitted for registration. |
|
AAFC HC (PMRA) NRCan 1. AAFC |
III. Generation of data to support the registration of reduced-risk and minor-use pesticides for the agricultural and agri-food sector and reduced-risk pesticides and biopesticides for forestry (a) Improving access to agricultural minor-use pesticides, and reduced-risk pesticides for agricultural use. |
$33.7M $12.0M Abase |
$6.5M $2.0M Abase |
Thirty-six pest-crop combinations will be identified at annual national stakeholder meeting hosted by AAFC. Manufacturer (registrant) written support will be obtained by July 2006 for each pest-crop pair, then sent to the PMRA for review by October with the majority by August (PSCR 3.1). Subsequently, data requirements (DACO) for each pest-crop pair will be issued by the PMRA to AAFC according to PMRA-established timeline (97 days from receipt) AAFC will convert DACOs to study plans by January 2007 and assign trials that complete the study plans, to contractors and collaborating AAFC personnel across Canada. Good laboratory practice trials require quality assurance oversight that is provided by contractors and AAFC Headquarters staff. Data generation from field trials in 2006 and laboratory analysis of residues proceeds to final report stage in the spring-summer of 2007 and are submitted to the PMRA. The PMRA provides a decision on use 247 days later. Total process takes about 36 months. |
| 2. HC (PMRA) | (a) Improving access to agricultural minor-use pesticides, and reduced-risk pesticides for agricultural use. | $20.8M | $4.0M |
Product evaluation work--review presubmission proposals from AAFC and provincial coordinators and issue data requirements. Register new minor crop uses, including minor use and reduced-risk products and uses. Harmonization work and regulatory projects-- Joint Reviews in collaboration with the U.S. EPA, AAFC and U.S. Department of Agriculture IR-4 Program, further work on crop groupings and on Maximum Residue Levels (MRL) promulgation. Increase communication and provide feedback to AAFC, to improve the quality and use of scientific rationales. |
| 3. NRCan | (b) Develop and facilitate the use of reduced-risk pesticides and biological pesticides for forestry. | $4.1 | $0.3 |
Review final reports of nine projects funded for three years and plan strategy and priorities for future funding. NRCan will continue work to integrate and coordinate activities with the other 5NR partners and stakeholders. The NRCan-CFS Minor Use Advisor hired under this fund will continue to work in collaboration with AAFC to facilitate registration of reduced risk/minor use pest control products against pest on outdoor woody ornamentals and forests. Coordinate and report on six projects for minor use pesticides in Canada. Support for the 2006 National Forest Pest Management Forum. Support for forest projects on reduce risk pest control products. |
| Results to be Achieved by Non-federal Partners: n/a | ||||
| Contact Information: Executive Director, PMRA | ||||
| Approved by: | Date Approved: | |||
| Horizontal Initiative: Federal Early Childhood Development (ECD) Strategy for First Nations and Other Aboriginal Children | Lead Department(s): Health Canada, First Nations Inuit Health Branch | |||
| Start Date: October 2002 | End Date: 2006-2007 and ongoing | |||
| Total Funding Allocated: As a result of an ECD Strategy announced in October 2002, $320 million over five years will be dedicated to enhancing various federal ECD programs. | ||||
| Description: The ECD Strategy for First Nations and Other Aboriginal Children was announced on October 31, 2002. The strategy provides $320 million over five years to: improve and expand existing ECD programs and services for Aboriginal children; expand ECD capacity and networks; introduce new research initiatives to improve understanding of how Aboriginal children are doing; and work towards the development of a "single window" approach to ensure better integration and coordination of federal Aboriginal ECD programming. | ||||
| Shared Outcome(s): The federal ECD Strategy complements the September 2000 First Ministers F/P/T ECD Agreement. It seeks to address the gap in life chances between Aboriginal and non-Aboriginal children by improving the developmental opportunities that Aboriginal children and their families are exposed to at an early age (0-6 years). These outcomes are shared by the following federal departments: Health Canada - First Nations Inuit Health Branch, Public Health Agency of Canada, Human Resources Development Canada, Social Development Canada, and Indian and Northern Affairs Canada. | ||||
| Governance Structure(s): Interdepartmental ECD ADM Steering Committee; Interdepartmental ECD Working Group. | ||||
| Federal Partners Involved in each program | Names of Programs | Total Allocation over 5 years ($ in Thousands) | Planned Spending for 2005-2006 | Expected Results for 2005-2006 |
|---|---|---|---|---|
|
1. Health Canada Electronic Link: |
(a) Aboriginal Head Start on Reserve (AHSOR) | $107,595 from 2002 MC (total for 2002-03 thru to 2006-07) | $21,519 (and ongoing) - committed in 2002 | Program expansion and enhancement |
|
Electronic Link: http://www.hc-sc.gc.ca/ fnihb/cp/fas_fae/ index.ht |
(b) Fetal Alcohol Spectrum Disorder - First Nations and Inuit Component (FASD-FNIC) | $70,000 (total for 2002-03 thru to 2006-07) | $15,000 (and ongoing) - committed in 2002 | Program expansion and enhancement |
| (c) Capacity building | $7,575 (total for 2002-03 thru to 2006-07) | $1,515 (and ongoing) - committed in 2002 | Program expansion and enhancement | |
|
Public Health Agency of Canada Electronic Link: |
(a) Aboriginal Head Start Urban and Northern (AHSUN) | $62,880 (total for 2002-03 thru to 2006-07) | $12,576 (and ongoing) - committed in 2002 | |
| 2. Human Resources Development Canada | (a) First Nations and Inuit Child Care Initiative (FNICCI) | $45,700 (total for 2002-03 thru to 2006-07) | $9,140 (and ongoing) - committed in 2002 | Program expansion and enhancement |
| (b) Research and knowledge | $21,200 (total for 2002-03 thru to 2006-07) | $4,240 (and ongoing) - committed in 2002 | Program expansion and enhancement | |
| 3. Indian Affairs and Northern Development | (a) Capacity Building and Indian Children Survey | ($5,050 - total for 2002-03 thru to 2006-07) | $1,010 (and ongoing) - committed in 2002 | Increased capacity |
| 4. Social Development Canada | (a) Non-applicable | |||
| Total | $320,000 | $65,000 | ||
| Results to be achieved by Non-Federal Partners (if applicable): | ||||
| Contact: Nicki Sims-Jones, Manager, ECD Strategy Unit, First Nations and Inuit Health Branch, Health Canada Postal Locator 1919B Tunney's Pasture, Ottawa Telephone: (613) 948-2589 Fax: (613) 946-4625 | ||||
| Approved by: Carolyn Harrison, Director, Children and Youth Directorate, First Nations and Inuit Health Branch, Health Canada, Postal Locator 1919B, Tunney's Pasture, Ottawa Telephone: (613) 948-5445 Fax: (613) 946-4625 | Date Approved: 01/10/05 | |||