Common menu bar links
Breadcrumb Trail
ARCHIVED - Patented Medicine Prices Review Board Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
SECTION II - ANALYSIS OF THE PROGRAM ACTIVITY BY STRATEGIC OUTCOME
Analysis by Program Activity
Strategic Outcome:
Prices charged by patentees for patented medicines sold in Canada are not excessive and Canadians are informed on pricing trends of medicines, as well as the R&D spending of pharmaceutical patentees.
Program Activity Name:
Patented Medicine Prices Review
Financial Resources ($ millions):
| Planned Spending | Authorities | Actual Spending |
| $11,475.0 | $11,924.8 | $7,432.4 |
Human Resources:
| Planned | Actual | Difference |
| 62.0 | 50 | 12 |
Priority 1: Compliance and Enforcement
Financial Resources ($ thousands):
| Planned Spending | Authorities | Actual Spending |
| $8,589.5 | $8,935.7 | 5,706.5 |
Human Resources:
| Planned | Actual | Difference |
| 43 | 37 | 6 |
Price Reviews
The PMPRB reviews pricing information filed pursuant to the Patented Medicines Regulations (Regulations) for all new and existing patented medicines sold in Canada, both prescription and non-prescription, to ensure that the prices charged by patentees are not excessive - i.e., that they comply with the Excessive Price Guidelines (Guidelines) established by the Board.4
These Guidelines are based on the price determination factors in subsection 85(1) of the Act which the Board must consider and were developed by the Board in consultation with stakeholders, including the provincial and territorial Ministers of Health, consumer groups and the pharmaceutical industry.
The price reviewed by the PMPRB is that charged by a patentee at the “factory gate”. This encompasses the prices charged in Canada by patentees for both prescription and non-prescription patented drugs, for human and veterinary use, to each class of customer5 in each province and territory.
In summary, the Guidelines provide that:
- New medicines that are new chemical entities are classified as either breakthrough/substantial improvement, moderate, or little or no therapeutic advantage over comparable medicines; new drug products can also represent a new presentation of an existing medicine in an existing or comparable dosage form (sometimes referred to as line extensions);
- Prices for new breakthrough patented drug products and those that bring a substantial therapeutic improvement are generally limited to the median of the prices charged for the same drug in the comparator countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States);
- Prices for new patented drug products that offer moderate, little or no therapeutic advantage over comparable medicines are limited to the highest price of comparable existing drugs used to treat the same disease or symptoms;
- Prices for new patented drug products that are line extensions of an existing medicine must bear a reasonable relationship to other strengths of the same or comparable dosage forms already on the Canadian market;
- After introduction, price increases are limited to changes in the Consumer Price Index (CPI); and
- The price of a patented drug product in Canada can never exceed the highest price for the same drug product in the foreign countries listed in the Regulations.
The expected result of the price review program is that all patentees' prices for new and existing patented medicines sold in Canada are reviewed in a timely and transparent manner and are in accordance with the Board's Excessive Price Guidelines.
The program activity supports the Government's priority of healthy Canadians by ensuring that Canadians have access to patented pharmaceutical products at prices that are not excessive.
The indicators that show that the PMPRB is achieving its expected results, and in turn contributing to its strategic outcome, are as follows:
- The prices of over 85% of patented medicines sold in Canada in calendar year 2007 were within the Guidelines;
- On average, price increases for existing patented medicines (0.1%) do not exceed and are well below changes in the Consumer Price Index (CPI) (2.1%);
- Enforcement measures (negotiation of Voluntary Compliance Undertakings [ VCUs ]; issuance of Notices of Hearings ) are taken to ensure prices are not excessive; and
- Canadian prices of patented drugs are, on average, only slightly above the median of international prices in the foreign countries listed in the Regulations, with the slight upward trend in Canadian prices relative to those in foreign countries largely due to the appreciating Canadian dollar and exchange rates.
Price Review of New Patented Drugs for Human Use
Sixty-four new patented drugs products6 (DINs) were introduced for human use in 2007. Of these, 20 medicines were new active substances, representing 34 DINs. By March 31, 2008, the Board had reviewed 53 of the 64 introduced. Of those, 47 were considered to be within the Guidelines while 6 appeared to exceed the Guidelines and were subject to ongoing investigations. At March 31, 2008, of the original 64 new drug products, 11 DINs remained under review.
Price Review of Existing Patented Drugs for Human Use
A total of 1,114 existing patented drug products (or DINs) were sold during 2007.7 Of these
- 975 DINs (87.5%) were within the Guidelines
- 14 DINs were subject to investigation due to introductory pricing
- 12 were opened in 2006
- 1 was opened in 2005
- 1 was opened in 2004
- 83 DINs were subject to investigations due to price increase
- 37 were opened in 2007
- 31 were opened in 2006
- 14 were opened in 2005
- 1 was opened in 2003
- 22 DINs were, or currently are, the subject of a hearing under section 83 of the Act. (For additional information see Quasi-judicial Activities - Hearings, on page 25.)
- 3 pertaining to Nicoderm
- 6 pertaining to Adderall XR
- 4 pertaining to Concerta
- 5 pertaining to Strattera
- 1 pertaining to Copaxone
- 1 pertaining to Penlac
- 1 pertaining to Quadracel
- 1 pertaining to Pentacel
- 20 DINs were still under review.
A summary of the review, compliance and investigation status of the new and existing patented drug products for human use in 2007 is provided in Table 1 below.
Table 1
| Patented Drug Products (DINs) for Human Use Sold in 2007 - Status of Price Review as of March 31, 2008 | |||
| New Drugs Introduced in 2006 | Existing Drugs | Total | |
| Total | 64 | 1,114 | 1,178 |
| Within Guidelines | 48 | 975 | 1,022 |
| Under Review | 11 | 20 | 31 |
| Under Investigation | 6 | 97 | 103 |
| Notice of Hearing | 0 | 22 | 22 |
Update of the Review of Existing Patented Medicine Prices reported in the 2006-2007 Departmental Performance Report
In the PMPRB's Performance Report for the fiscal year ending March 31, 2007, it was reported that, of the 1,082 existing patented drug products for human use sold in 2006, the prices of 17 were still under review. The results of those reviews concluded that: 6 drug products were within the Guidelines; 4 drug products were priced at levels that appeared to exceed the Guidelines and therefore investigations were initiated; and 7 are still under review. In addition one further drug has been added to those under review due to failure to file at the time when the drug product came under the PMPRB's jurisdiction.
The PMPRB also reported that 65 DINs were under investigation. Of those, 17 investigations have been concluded: in 13 cases the prices were ultimately found to be within the Guidelines; and for 4 cases, VCUs were approved: Forteo, Octreo Scan, Vaniqa, and Zemplar, the latter having been subject to a Notice of Hearing (See Voluntary Compliance Undertakings on page 22).
Patented Drugs for Veterinary Use
The Board has adopted a policy for the review of patented veterinary drug products that differs somewhat from that for human drugs. Board Staff reviews the introductory prices of new patented veterinary drug products according to the current Guidelines and determines whether or not the price is excessive. In subsequent years, however, veterinary drug product prices are subject to formal review only when a complaint is received. Patentees are required to maintain price information for all reporting periods in which the product is under the Board's jurisdiction, but only need to file this information with the PMPRB upon request following a complaint. No complaints were received in 2007. Last year we reported that one patented drug product for veterinary use was under introductory price review, and it remained under review as of March 31, 2008.
In 2007, 7 new patented drug products for veterinary use were reported to the PMPRB. These are under review. Summary reports of the price reviews of drug products for veterinary use are made available on the PMPRB's Web site under Regulatory; Patented Medicines; Reports on New Patented Drugs for Veterinary Use.
Enforcement Measures
Voluntary Compliance Undertakings
A Voluntary Compliance Undertaking (VCU) is a written undertaking by a patentee to adjust the price of a patented drug product to conform to the Excessive Price Guidelines (Guidelines).
Under the Compliance and Enforcement Policy, patentees are given an opportunity to submit a VCU when Board Staff concludes, following an investigation, that the price at which the patentee is selling or has sold the drug product in Canada appears to have exceeded the Guidelines.
Approval of a VCU by the Chairperson is an alternative compliance mechanism to the commencement of formal proceedings through the issuance of a Notice of Hearing. Under the PMPRB's Compliance and Enforcement Policy, a VCU can also be submitted following the issuance of a Notice of Hearing. A VCU submitted at this point must be approved by the Hearing Panel.
In 2007-2008, VCUs were approved for eight patented medicines:8
Airomir, 3M Canada Company
Airomir is used for the treatment of asthma, chronic bronchitis, and other breathing disorders.
On May 14, 2007, the Hearing Panel approved a VCU agreed to by 3M Canada Company (3M Canada) and Board Staff, for the payment in full of revenues alleged by Board Staff to have been excessive, totaling $485,498.58, derived from January 1, 2004 to December 29, 2006. The hearing into the price of Airomir, commenced by the issuance of a Notice of Hearing on February 20, 2006, was concluded with the approval of the VCU. 3M Canada met the terms of the VCU.
Dovobet, LEO Pharma Inc.
Dovobet is a dermatological drug required for bringing psoriasis under control.
On January 19, 2008, the Chairperson of the Board approved a VCU submitted by LEO Pharma Inc., for the medicine Dovobet. A Board Order issued on September 17, 2007, following a hearing, required LEO Pharma to price Dovobet at a non-excessive level, and to offset the excess revenues derived from the sale of Dovobet in Canada from 2002 through to December 2005. (For more information on the hearing in this matter, see Quasi-judicial Activities - Hearings, on page 25.) For the period January 1, 2006 through December 31, 2006, Board Staff calculated the MNE price in accordance with the Board Order. In 2006, the average transaction price (ATP) of Dovobet exceeded the 2006 price, resulting in excess revenues of $870,425.68. To offset these excess revenues, LEO Pharma submitted a VCU and made a payment in full to the Government of Canada.
Forteo, Eli Lilly Canada Inc.
Forteo is indicated for the treatment of postmenopausal women with severe osteoporosis who are at high risk of fracture or who have failed or are intolerant to previous osteoporosis therapy; and to increase bone mass in men with primary or hypogonadal severe osteoporosis who have failed or are intolerant to previous osteoporosis therapy.
On June 28, 2007, the Chairperson accepted a VCU for Forteo submitted by Eli Lilly Canada Inc. (Lilly). The VCU included a reduction of the price of Forteo below the MNE price for 2007 in order to offset excess revenues. In the event that all excess revenues had not been offset by December 31, 2007, Lilly had undertaken to make a payment to the federal government in the amount of the remainder of the excess revenues that had not been offset. Excess revenues were offset by December 31, 2007.
Lantus, sanofi-aventis Canada Inc.
Lantus (insulin glargine) is indicated for once-daily subcutaneous administration in the treatment of adult patients with Type 1 or Type 2 diabetes mellitus and pediatric patients (age 6-17 years) with Type 1 diabetes mellitus who require basal (long-acting) insulin for the control of hyperglycemia.
On March 14, 2008, the Chairperson of the Board approved a VCU submitted by sanofi-aventis Canada Inc. (sanofi-aventis) for the medicine Lantus. In addition to reducing the price of Lantus to a non-excessive level, sanofi-aventis offset the cumulative excess revenues it received from sales of Lantus as of September 18, 2006 by making a payment to the Government of Canada in the amount of $694,239.50 and by reducing the price of another medicine, ALTACE HCT. In the event that the full amount of excess revenues, totaling $3,969,554.83, has not been completely offset by December 31, 2008, sanofi-aventis has undertaken to make a further payment to the Government of Canada.
OctreoScan, Bristol-Myers Squibb Canada Co.
OctreoScan is a radiopharmaceutical agent used for the diagnosis of brain diseases and tumors.
On September 19, 2007, the Chairperson of the Board accepted a VCU for OctreoScan submitted by Bristol-Myers Squibb Medical Imaging, a Division of Bristol-Myers Squibb Canada Co. (Bristol-Myers Squibb). In addition to reducing the price of OctreoScan to a non-excessive level, Bristol-Myers Squibb offset the excessive revenues accrued, in the amount of $387,181.87, by making payments to the hospitals that purchased OctreoScan and by making a payment to the Government of Canada for the remaining excess revenues in the amount of $7,439.82.
Risperdal Consta, Janssen-Ortho Inc.
Risperdal Consta is a new formulation of an existing compound (risperidone) indicated for the management of the manifestations of schizophrenia and related psychotic disorders.
On June 7, 2007, the Hearing Panel approved a VCU agreed to by Janssen-Ortho Inc. and Board Staff to, among others, reduce the price of Risperdal Consta to a non-excessive level and to offset excess revenues in the amount of $4,386,172.99. By Order of the Board, the proceeding that was commenced with the issuance of a Notice of Hearing on January 30, 2006, was thereby concluded. Janssen-Ortho Inc. met the terms of the VCU.
Vaniqa, Barrier Therapeutics Canada Inc.
Vaniqa (eflornithine hydrochloride) is indicated for slowing the growth of unwanted facial hair in women. It is recommended as an adjunct to any hair removal technique.
On February 28, 2008, the Chairperson of the Board approved a VCU submitted by Barrier Therapeutics Canada Inc., for the medicine Vaniqa. Barrier reimbursed the excess revenues accrued over the period of November 2005 to December 2007, by making a payment to the Government of Canada, in the amount of $70,860.59. Vaniqa is no longer sold in Canada.
Zemplar, Abbott Laboratories Limited
Zemplar is indicated for the prevention and treatment of secondary hyperparathyroidism associated with chronic renal failure.
On September 26, 2007, the Hearing Panel approved a VCU agreed to by Abbott Laboratories Limited (Abbott) and Board Staff to ensure that, among others, the price of Zemplar is not excessive and to offset alleged excess revenues in the amount of $58,741.67. The Chairperson had issued a Notice of Hearing on July 24, 2007, pertaining to the allegations of Board Staff that Zemplar had been, and was being, sold by Abbott at prices exceeding those indicated by the Board's Guidelines. With the Hearing Panel's approval of the above-mentioned VCU, which proposed to resolve all issues raised by the Notice of Hearing, by Order of the Board, the proceeding was thereby concluded. Abbott met the terms of the VCU.
Quasi-judicial Activities - Hearings
Under section 83 of the Act, the Board can hold a public hearing to determine whether a patented medicine is being or has been sold at an excessive price and, if it finds that the price is or was excessive, it may issue an Order to reduce the patentee's price and to offset the excess revenues.
On April 1, 2007, there were eleven ongoing hearings carried over from previous years. Between that date and March 31, 2008 the Board issued one Notice of Hearing into the matter of Apotex and its status as a patentee under the PMPRB's jurisdiction. It also initiated proceedings into the matter of Celgene Corporation and the medicine Thalomid with respect to its jurisdiction over the price of the medicine. Of these 14 hearings, three were resolved by way of VCUs: Airomir, Riserpdal Consta and Zemplar. More details on these VCUs are available in the VCU section of this report. Board Orders, concluding the proceedings, were issued in the Dovobet and Copaxone matters (although the Order for Copaxone was not issued until fiscal year 2008-2009). The Thalomid matter was also concluded when the Hearing Panel ruled that it has jurisdiction over the price of the medicine.
Adderall XR, Shire BioChem Inc.
Adderall XR is a medicine indicated for the treatment of Attention Deficit Hyperactivity Disorder.
The Board issued a Notice of Hearing in this matter on January 18, 2006. The Hearing Panel issued its decision on the merits on April 10, 2008. Although not during the period of this review, the Board issued an Order on August 27, 2008, concluding these proceedings.
On December 15, 2006, the Hearing Panel issued a decision dismissing Shire's motion for an order that the Board amend its Notice of Hearing to limit its inquiry to the period following the date of issuance of Shire's patent 2,348,090, namely, April 13, 2004. Shire filed an application for judicial review with the Federal Court. The FC issued its decision on December 19, 2007, dismissing the matter. Shire has appealed the FC decision. The Federal Court of Appeal has not yet heard the parties on this issue.
Airomir, 3M Canada Company
Airomir is used for the treatment of asthma, chronic bronchitis, and other breathing disorders.
The proceeding into the matter of 3M Canada Company and the price of Airomir commenced with the issuance of a Notice of Hearing on February 20, 2006 and was concluded with the approval of a Voluntary Compliance Undertaking (VCU) on May 14, 2007.
Apotex Inc.
The Board issued a Notice of Hearing in the matter of Apotex Inc. on March 3, 2008, concerning that company's status as a patentee and the information filing requirements pursuant to the Patent Act and the Patented Medicines Regulations. The Hearing Panel is scheduled to hear this matter on October 6, 2008.
Concerta, Janssen-Ortho Inc.
Concerta is indicated for the treatment of Attention Deficit Hyperactivity Disorder.
The Board issued a Notice of Hearing in this matter on July 24, 2006. The Board's decision is pending.
Note: Janssen-Ortho was granted the status of intervener in the judicial review application launched by Shire with regard to the Board's December 15, 2006 decision (Shire and the issue of pre-patent, as described under Adderall XR above). Janssen-Ortho has also appealed the December 19, 2007 Fe d eral Court decision dismissing the case.
Copaxone, Teva Neuroscience G.P.-S.E.N.C.
Copaxone is indicated for use in ambulatory patients with Relapsing-Remitting Multiple Sclerosis to reduce the frequency of relapses.
The Board issued a Notice of Hearing into the matter of Copaxone on May 8, 2006. After hearing the parties, the Hearing Panel issued its decision and reasons in this matter on
February 25, 2008, including instructions that the parties file a proposed Board Order. The Panel received separate submissions on a proposed Board Order. In its Order issued on May 12, 2008, having found that Copaxone had been sold at an excessive price, the Board required Teva to reimburse $2,417,223.29 in excess revenues. Teva Neuroscience has filed a Notice of Application with the FC seeking judicial review. A hearing date has not yet been announced.
Dovobet, LEO Pharma Inc.
Dovobet is a dermatological drug administered to bring psoriasis under control.
The Board issued a Notice of Hearing in the matter of LEO Pharma Inc. and the medicine Dovobet on November 29, 2004. This matter was concluded with the issuance of a Board Order, on September 17, 2007, requiring LEO Pharma to price Dovobet at a non-excessive level, and to offset the excess revenues derived from the sale of Dovobet in Canada from 2002 through to December 2005 in the amount of $3,736,398.71.
Nicoderm, Hoechst Marion Roussel Canada Inc.
Nicoderm is indicated for smoking cessation.
The Board issued a Notice of Hearing in this matter in April 1999. Following extended proceedings before the Federal Court, the matter was returned before the Board. The Hearing Panel heard the parties on the resolution of this matter on July 3, 2008, following a joint submission by the parties to terminate the hearing. In its decision of July 21, 2008, the Board indicated that it was not persuaded that this proceeding should be discontinued and instructed the parties to continue with the proceeding and issued a schedule to that effect. The hearing will resume on November 21, 2008.
Penlac, sanofi-aventis Canada Inc.
Penlac is indicated as part of a comprehensive nail management program in immunocompetent patients with mild to moderate onychomycosis without lunula involvement.
The Board issued a Notice of Hearing in this matter on March 26, 2007. Hearing sessions were initiated in June 2007 and continued in 2008. The hearing in this matter is to resume on December 8, 2008.
Quadracel and Pentacel, sanofi pasteur Limited
Quadracel is indicated for the primary immunization of infants, at or above the age of 2 months, and as a booster in children up to their 7 th birthday against diphtheria, tetanus, whooping cough (pertussis) and poliomyelitis.
Pentacel is indicated for the routine immunization of all children between 2 and 59 months of age against diphtheria, tetanus, whooping cough (pertussis), poliomyelitis and haemophilus influenzae type b disease. It is sold in Canada in the form of a reconstituted product for injection combining one single dose vial of Act HIB (Lyophilized powder for injection) and one single (0.5 mL) dose ampoule of Quadracel (suspension for injection).
The Board issued a Notice of Hearing in this matter on March 27, 2007. Following the Hearing Panel's decision of November 26, 2007 denying sanofi pasteur's Motion that the Panel replace its counsel in this proceeding, sanofi pasteur filed a judicial review application with the FC. The application for judicial review was dismissed. The Panel reconvened this hearing on June 13, 2008. The hearing will resume on November 25, 2008.
Risperdal Consta, Janssen-Ortho Inc.
Risperdal Consta is a new formulation of an existing compound (risperidone) indicated for the management of the manifestations of schizophrenia and related psychotic disorders.
The Board issued a Notice of Hearing in the matter of Janssen-Ortho Inc. and the medicine Risperdal. Consta on January 30, 2006. The matter was concluded on June 7, 2007, with the Hearing Panel's approval of a VCU agreed to by Janssen-Ortho Inc. and Board Staff to, among others, reduce the price of Risperdal Consta to a non-excessive level and offset excess revenues in the amount of $4,386,172.99. For more information on the VCU, see Voluntary Compliance Undertakings, on page 22.
Strattera, Eli Lilly Canada Inc.
Strattera is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 years of age and over, adolescents and adults.
The Board issued a Notice of Hearing in this matter on December 15, 2006. The h earing will resume on October 25, 2008.
Thalomid, Celgene Corporation
Thalomid does not have a Notice of Compliance but patients in Canada have been purchasing Thalomid from Celgene in the United States since 1995 through Health Canada's Special Access Programme. Thalomid is used to slow the progress of multiple myeloma, a form of cancer.
On August 23, 2007, a Hearing Panel of the Board heard submissions from Celgene Corporation and Board Staff on the Board's jurisdiction in the matter of the price of Thalomid. In its decision of January 21, 2008, the Board ruled that it has jurisdiction over the price of Thalomid. Celgene Corporation filed a Notice of Application with the Federal Court for a judicial review of the Panel's decision. A hearing date has not yet been announced.
Zemplar, Abbott Laboratories Limited
Zemplar is indicated for the prevention and treatment of secondary hyperparathyroidism associated with chronic renal failure.
The Board issued a Notice of Hearing in this matter on July 24, 2007. The matter was concluded on September 26, 2007, with the approval of a VCU agreed to by Abbott Laboratories Limited and Board Staff to ensure that, among others, the price of Zemplar is not excessive and to offset alleged excess revenues in the amount of $58,741.69. For more information on the VCU, see Voluntary Compliance Undertakings on page 22.
Amendments to the Patented Medicines Regulations
The regulatory amendments to the Patented Medicines Regulations, 1994 (Regulations) were registered on March 6, 2008 and received final publication in the Canada Gazette, Part II, on March 19, 2008. These amendments modernize the Regulations by increasing efficiency and timeliness in the price review process for patented medicines.
This regulatory initiative began in January 2005 with the publication of a Notice and Comment proposal to amend the Regulations, followed by the initial pre-publication of the proposed regulatory amendments in the Canada Gazette, Part I, on December 31, 2005. Following extensive stakeholder consultations, a revised regulatory package was pre-published in the Canada Gazette, Part I, on October 6, 2007. Several stakeholder submissions were received during the second pre-publication consultation period. These submissions remain posted on the PMPRB Web site for the information of all interested parties.
In response to stakeholder concerns, the final amendments contained two changes from the proposed amendments which were pre-published on October 6, 2007:
- the proposed requirement that patentees identify the type of reductions used in the calculation of average price per package or net revenue from sales was removed; and
- the date of the coming into force of the electronic filing requirement was changed from January 1, 2009 to July 1, 2008, as the electronic forms no longer needed to be revised to accommodate the filing of reduction information by type.
The amendments also put into place the following changes regarding reporting information to the PMPRB:
Regulatory Filing
- Information identifying the medicine (Form 1) shall now be accompanied by the product monograph for the medicine or, if a notice of compliance (NOC) has not been issued in respect of the medicine, by information similar to that contained in a product monograph.
- Information identifying the medicine (Form 1) shall now be provided no later than the earlier of seven days after the day on which the first NOC is issued in respect of the patented drug product, and seven days after the day on which the product is first offered for sale in Canada.
Patented Prescription Drug Products
- Where a patented prescription drug product is for human use, information on the prices of the product (Form 2) shall now be provided for the day on which the product is first sold in Canada, within 30 days after that day; this replaces the previous requirement that information be provided on the first 30 day sales.
Veterinary and Over-the-Counter Drug Products
- For veterinary and over-the-counter drug products, information on the prices of the product (Form 2) shall now be provided on a complaints-based approach, wherein a patentee shall provide to the Board the necessary information for each six-month period, beginning on January 1 and July 1 of each year, within 30 days after the date on which the Board sends a request in response to a complaint respecting the price of the product, and during the two years following the request, within 30 days after each six-month period.
Electronic Filing
- Patentees are now required to provide information for all three forms (1, 2 and 3, which pertains to information on sales and research and development [ R&D ] expenditures ) to the Board using specified electronic documents in their original format and file type, bearing the electronic signature of an authorized individual, certifying that the information set out in the documents is true and complete.
Patentees were required to comply with the amended Regulations as of their final publication on March 19, 2008, with the exception of the electronic filing requirement which must be complied with as of July 1, 2008 for the July-to-December 2008 reporting period. Board Staff provided information sessions to patentees in May and June 2008 to explain how to fully comply with the regulatory amendments.
Review of the Board's Excessive Price Guidelines
Throughout 2007-2008, the Board was actively engaged in the review of its Excessive Price Guidelines (Guidelines) to ensure that they remain relevant and appropriate in the context of the current pharmaceutical environment.
The activities undertaken as part of the Guidelines review in 2007-2008 built on the work of the previous year, which began with the release of the Discussion Guide on the Board's Excessive Price Guidelines in May 2006. This was followed by a series of national consultations in November 2006, where the Board met with close to 140 members of various stakeholders groups.
On May 31, 2007, the Board released a Stakeholder Communiqu� outlining its preliminary decisions and directions on the issues under consultation to date, as well as the next steps over the remainder of the year. The Stakeholder Communiqu� also signaled the launch of three new Working Groups: one to determine definitions and levels of evidence for various categories of therapeutic improvement; one to determine appropriate therapeutic comparators for domestic drug products in other countries; and one to seek input from experts on how to define the costs of making and marketing a drug product (subsection 85(2) of the Patent Act).
In the midst of the more general review of the Guidelines, in March 2007, the Federal Court (FC) issued a decision in response to a judicial review application in the matter of LEO Pharma Inc. and the price of the patented medicine Dovobet. In April 2007, the PMPRB published an article in its NEWSletter informing stakeholders of the implications and impacts of the FC decision. Stakeholders were instructed that all benefits (as defined by the Patented Medicines Regulations, 1994 (Regulations) in subsections 4(4) and 4(5), hereinafter referred to simply as “benefits”, must now be included in the calculation of the average price of a patented medicine. Communiqu�s on this matter were issued in May and June, indicating the Board's desire to further consider and consult with stakeholders.
Significant concern was expressed by the patented pharmaceutical industry regarding the potential disincentives the decision would have on the willingness of companies to offer, or continue to provide, various benefits to their customers. Representatives of the innovative pharmaceutical and biotechnology industries were then given the opportunity to comment on the implications of the FC decision during face-to-face meetings with the Board during the summer of 2007.
On September 10-12, 2007, the Board held a series of bilateral consultation meetings with stakeholder groups, representing sectors of the pharmaceutical industry (innovative, biotechnology and generic), federal/provincial/territorial (F/P/T) governments and consumers. The purpose of these meetings was to provide participating stakeholders with an opportunity to raise their comments directly with Board Members in relation to the issues regarding reporting of benefits, as well as other concerns they may or may not have raised in previous consultations on the Guidelines. In October 2007, the Board released a communiqu� indicating it would not insist on full reporting of benefits until January 1, 2009, to permit the opportunity for further consideration with stakeholders.
On January 31, 2008, the Board released the Discussion Paper - Options for Possible Changes to the Patented Medicines Regulations, 1994 and the Excessive Price Guidelines. In total, the Board received 43 submissions from a wide range of stakeholders. In keeping with the Board's commitment to openness and transparency, all stakeholder submissions can be found on the PMPRB Web site.
In early April 2008, the Board also received the final reports of both the Working Group on Therapeutic Improvement, and the Working Group on International Therapeutic Class Comparison. Building on the efforts of the previous two Working Groups, the Board launched an additional Working Group to develop advice and options for possible changes to the PMPRB's price tests.
In addition, throughout this period a sectoral Working Group involving representatives of the Canadian Generic Pharmaceutical Association and Board Staff met to consider unique market issues faced by patented generic drug products and to develop options and advice for the Board on possible selected tailored Guidelines, which would respond to these challenges. This Working Group's report can be found on PMPRB's Web site, along with reports on the above four work areas.
Recognizing that this first major review of the Guidelines since 1994 may create a degree of uncertainty for patentees and other stakeholders regarding the future of the price review process, the Board is committed to ongoing open communication through its NEWSletter, its Web site and other means, as appropriate. In addition, the Board released a Communiqu� on August 18, 2008, summarizing the information patentees will need to report on beginning with the January-June 2009 period and highlighting the next steps in the consultations on the Guidelines, including a Notice and Comment on draft revised Guidelines that was released on August 20, 2008.
Priority 2: Report on Pharmaceutical Trends
Financial Resources ($ thousands):
| Planned Spending | Authorities | Actual Spending |
| $2,885.5 | $2,989.1 | $1,725.9 |
Human Resources:
| Planned | Actual | Difference |
| 19 | 13 | 6 |
Price Trends
The PMPRB's second priority is to report on pharmaceutical price trends relating to all medicines, and on R&D spending by pharmaceutical patentees. This priority contributes to informed decisions and policy-making among stakeholders.
Section 100 of the Patent Act (Act) requires the Board to annually submit to the Minister of Health a report on its activities during the preceding calendar year. The report must include a summary of pricing trends in the pharmaceutical industry, and patentees' expenditures on research and development as a proportion of total revenues from sales of medicines in Canada. The Minister is required to table the report before Parliament. The PMPRB's 2007 Annual Report was tabled before Parliament on June 18, 2008.
The PMPRB uses the Patented Medicine Price Index (PMPI) to monitor and report on trends in prices of patented drugs. The PMPI is a price index measuring the average year-over-year change in the ex-factory prices of patented drugs sold in Canada. The PMPI does not measure changes in the utilization of patented drugs: a quantity index, the PMQI, is calculated for this purpose. The PMPI does not measure the cost-impact of changes in prescribing patterns or the introduction of new medicines. By design, the PMPI isolates the component of sales growth attributable to changes in the prices of patented drugs.
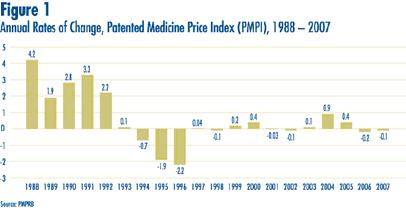
Figure 1 provides year-over-year changes in the PMPI for the years 1988 through 2007. As measured by the PMPI, prices of patented drugs declined on average by 0.1% between 2006 and 2007.
Comparison of PMPI and CPI
Section 85 of the Act provides that, among other factors, the PMPRB shall consider changes in the Consumer Price Index (CPI) in determining whether the price of a patented drug is excessive.
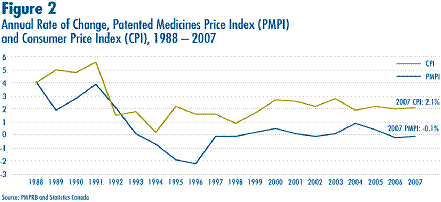
Figure 2 plots year-over-year rates of change in the PMPI against corresponding changes in the CPI. Inflation, as measured by the CPI, has exceeded the average increase in patented drug prices almost every year since1988. This pattern continued in 2007, with the CPI rising by 2.1% as the PMPI fell by 0.1%. That the PMPI has not kept pace with the CPI is not surprising. The Board's Guidelines allow the price of a patented drug to rise by no more than the CPI over any three-year period. (The Guidelines also impose a cap on year-over-year price increases equal to one-and-one-half times the current year rate of CPI-inflation.)
This effectively establishes CPI-inflation as an upper bound on the rate at which the PMPI may rise over any period of three years. Increases in the PMPI normally do not reach this upper bound because many patentees do not raise their prices by the full amount permitted under the Guidelines.
Price Change by Therapeutic Class
Table 2 provides average rates of price change among patented drugs at the level of major therapeutic classes. Results in this Table were obtained by applying the PMPI methodology to data segregated by the World Health Organization's (WHO) Atomic Therapeutic Chemical (ATC) Level I class. The last column provides a decomposition of overall PMPI change, with each entry representing the component of the overall change attributable to drugs in the corresponding therapeutic class. By this measure, drugs treating blood and bloodforming organs were the largest contributor (in absolute magnitude) to overall price change in 2007.
Table 2
| Change in the Patented Medicine Price Index (PMPI) by Major Therapeutic Class, 2007 | |||
| Therapeutic Class | Share of Sales (%) | PMPI Change: 2006 to 2007 (%) |
Contribution to Overall Change (%) |
| A: Alimentary Tract and Metabolism | 13.0 | -0.5 | -0.1 |
| B: Blood and Blood Forming Organs | 7.2 | -2.2 | -0.2 |
| C: Cardiovascular System | 25.1 | 0.2 | 0.0 |
| D: Dermatologicals | 1.0 | 0.3 | 0.0 |
| G: Genito-urinary System and Sex Hormones | 3.4 | 0.7 | 0.0 |
| H: Systemic Hormonal Preparations | 0.89 | -1.1 | 0.0 |
| J: General Antiinfectives for Systemic use; and P: Antiparasitic Products9 |
9.5 | 0.6 | 0.1 |
| L: Antineoplastics and Immunomodulating Agents | 13.6 | 0.0 | 0.0 |
| M: Musculo-skeletal System | 4.0 | 0.1 | 0.0 |
| N: Nervous System | 13.0 | -0.4 | -0.1 |
| R: Respiratory System | 7.7 | 0.7 | 0.0 |
| S: Sensory Organs | 1.3 | -0.5 | 0.0 |
| V: Various | 0.5 | -1.0 | 0.0 |
| All Therapeutic Classes | 100.0* | -0.1 | -0.1 |
| Source: PMPRB * Values in this column may not add to 100.0 due to rounding. |
|||
Price Change by Class of Customer
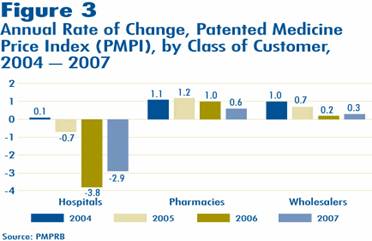
Figure 3 presents average rates of price change by class of customer10. These results were obtained by applying the PMPI methodology to data on sales of patented drugs made specifically to hospitals, to pharmacies and to wholesalers11.
Rates of 2006-to-2007 price change ranged from -2.9% for sales to hospitals to 0. % for direct sales to pharmacies. Not surprisingly, the rate of price change for sales to wholesalers (which accounts for about three-quarters of all sales) is closest to the overall change in the PMPI. Note that in all customer classes, rates of price change were substantially less than CPI-inflation of 2.1%.
It is clear from Figure 3 that the slight decline in the overall PMPI was the result of falling prices paid by hospital customers: a PMPI covering only sales to pharmacies and wholesalers would have risen by approximately 0.3% between 2006 and 2007.
Price Change by Province/Territory
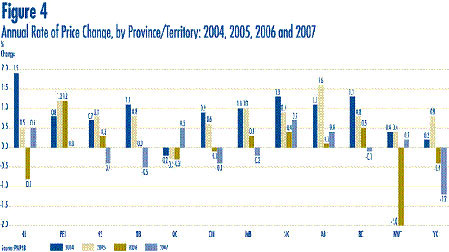
Figure 4 presents average rates of price change by province/territory. These results were obtained by applying the PMPI methodology to data segregated by the province/territory in which the sale took place. Rates of price change range from -1.2% in the Yukon to 0.7% in Saskatchewan. Average price increases in five of the twelve provincial/territorial jurisdictions were offset by the modest decline in Ontario, resulting in the average national price decrease of 0.1 %.
Price Change in Canada and Comparator Countries
In accordance with the Act and the Regulations, patentees must report publicly available ex-factory prices of patented drugs in seven foreign countries. These countries are: France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States. The PMPRB uses this information to conduct the international price comparison tests specified in the Guidelines and to compare the Canadian prices of patented drugs with those in other countries.
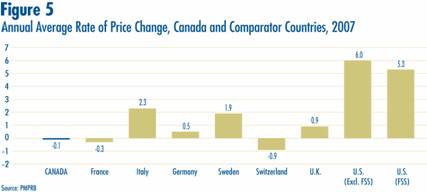
Figure 5 gives average annual rates of price change for Canada and each of the seven comparator countries. These results were obtained by applying the PMPI methodology (with weights based on Canadian sales patterns) to international price data submitted to the PMPRB. Note that two results are presented for the U.S. The first of these is restricted to published U.S. “market” prices, typically wholesale acquisition costs. The second incorporates prices from the U.S. Federal Supply Schedule (FSS).
Five of seven comparator countries registered overall price increases between 2006 and 2007, the exceptions being France and Switzerland. Switzerland saw the largest average decline (-0.9%). In contrast, U.S. prices rose by nearly 6%.
Bilateral Comparison of Foreign Prices to Canadian Prices
Table 3 provides bilateral comparisons of prices in each of the PMPRB's seven comparator countries to corresponding Canadian prices. Focusing on the results with currency conversion at market exchange rates (and calculated as a geometric mean), it appears that Canada's prices of patented drugs are slightly higher than prices in all countries other and the U.S. Prices in Italy and France are, on average, appreciably less than Canadian prices. As in previous years, 2007 U.S. prices were substantially higher than prices in Canada and every other comparator country.
Table 3
| Average Foreign-to-Canadian Price Ratios, Bilateral Comparisons, 2007 | ||||||||
| (i) At Market Rates | Can | Fra | Ita | Ger | Swe | Swi | U.K. | U.S. |
| Geometric Mean | 1.00 | 0.85 | 0.77 | 0.98 | 0.94 | 0.99 | 0.98 | 1.64 |
| Arithmetic Mean | 1.00 | 0.90 | 0.82 | 1.07 | 0.99 | 1.06 | 1.03 | 1.76 |
| Number of DINs | 1,145 | 748 | 744 | 840 | 816 | 797 | 835 | 985 |
| Net Revenues ($M) | 12,3437 | 10,620 | 10,339 | 10,711 | 10,843 | 11,101 | 11,179 | 11,4790 |
| (ii) At PPPs | Can | Fra | Ita | Ger | Swe | Swi | U.K. | U.S. |
| Geometric Mean | 1.00 | 0.76 | 0.72 | 0.92 | 0.78 | 0.76 | 0.94 | 1.71 |
| Arithmetic Mean | 1.00 | 0.81 | 0.78 | 1.00 | 0.82 | 0.82 | 0.88 | 1.85 |
| Number of DINs | 1,145 | 748 | 744 | 840 | 816 | 797 | 835 | 985 |
| Net Revenues ($M) | 12,3437 | 10,620 | 10,339 | 10,711 | 10,843 | 11,101 | 11,179 | 11,4790 |
Multilateral Price Comparisons
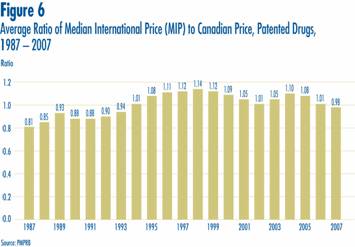
Focusing again on results at market exchange rates (and obtained using the geometric mean), the average Median International Price (MIP)-to-Canadian price ratio stood at 0.98 in 2007. By this measure, MIPs were on average slightly less than corresponding Canadian prices. Last year's Annual Report gave a value of 1.01 for 2006, indicating MIPs were slightly higher than Canadian prices. Figure 6 puts this result in historical perspective. MIPs were on average 19% less than corresponding Canadian prices in 1987. By 1998, MIPs were on average 14% higher than Canadian prices. The average MIP-to-Canadian price ratio had remained above parity from 1994 through to 2006.
Utilization of Patented Drugs
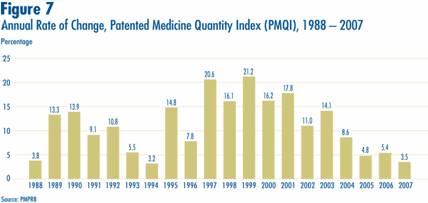
The price and sales data used to calculate the PMPI also allow the PMPRB to examine trends in the quantities of patented drugs sold in Canada. The PMPRB maintains the Patented Medicine Quantity Index (PMQI) for this purpose. Figure 7 displays average rates of utilization growth, as measured by the PMQI, from 1988 through 2007. These results confirm that growth in the utilization of patented drugs has been the primary source of rising sales, with rates of utilization growth roughly tracking rates of sales growth in recent years. This pattern continued in 2007, with utilization of patented drugs growing by 3.5%. (Sales growth also slowed in 2007 compared with previous years and stood at 3%.)
Canadian Sales in the Global Context
IMS Health regularly reports on patentees' sales to the retail sector across a wide range of countries. IMS reports that, in 2007, sales amounted to $450.3 billion among major markets.12 Figure 8 shows how this amount was distributed among these markets. Drug sales in Canada accounted for 3.8% of total major-market sales. The U.S. market is by far the largest, with drug sales nearly equal to the combined sales of all other major markets.
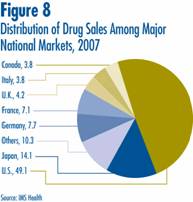
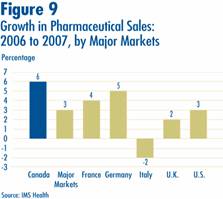
Figure 9 gives rates of 2007-over-2006 sales growth for individual major markets. Based on IMS data, Canadian sales growth exceeded growth observed in all other comparator countries including the U.S.
Analysis of Research and Development Expenditures
With the adoption of the 1987 amendments to the Patent Act (Act), Canada's Research-Based Pharmaceutical Companies (Rx&D) made a public commitment at that time that brand name manufacturers would increase their annual research and development (R&D) expenditure to 10% of sales revenue by 1996. Under the Act, the PMPRB monitors and reports on R&D spending, but has no regulatory authority over the amount or type of research spending by patentees.
The Act requires each patentee to report its revenue from sales of drugs (including revenue from sales of non-patented drugs and from licensing agreements) and R&D expenditure in Canada related to medicines. The Regulations require that R&D data submitted to the PMPRB be accompanied by a certificate stating that the submitted information is “true and correct”. The PMPRB does not audit submissions, but it does review submitted data for anomalies and inconsistencies, seeking corrections or clarifications from patentees where necessary. To confirm that Board Staff has correctly interpreted submitted data, each patentee is given the opportunity to review and confirm the accuracy of its own R&D-to-sales ratio before publication of the Board's Annual Report. Companies without sales of patented medicines in Canada need not report on their R&D activity. For this reason, as new patents are granted and others expire, the set of companies required to file R&D data may change from year to year.
In 2007, a total of 82 companies selling patented human and veterinary drug products filed reports on their R&D expenditure. Of these, 35 were members of Canada's Research Based Pharmaceutical Companies (Rx&D).
Sales Revenue
For reporting purposes, sales revenue is defined as all revenue from sales in Canada of medicines and from licensing agreements (e.g., royalties and license fees from sales in Canada by licensees).
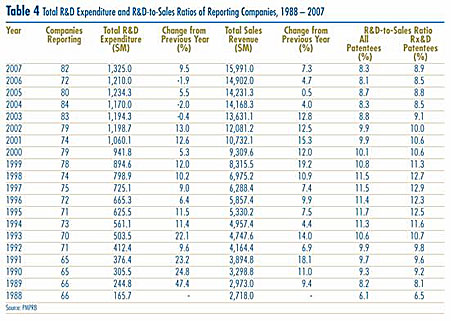
As shown in Table 4, patentees reported total 2007 sales revenue of $15.9 billion, up 7.3% from 2006. Sales revenue reported by Rx&D members was $13.4 billion, accounting for 83.7% of the total. Less than 1% of reported sales revenue was generated by licensing agreements.
R&D Expenditure
As shown in Table 4, total R&D expenditure reported by patentees was $1,325.0 million in 2007, an increase of 9.5% over 2006. Rx&D members reported R&D expenditure of $1,184.0 million in 2007, an increase of 24.7% over last year. Rx&D members accounted for 89.4% of all reported R&D expenditure.
Patentees that were not members of Rx&D reported R&D expenditure of $141 million in 2007, a decrease of 45.9% over last year.
R&D-to-Sales Ratios
The ratio of R&D expenditure to sales revenue among all patentees was 8.3% in 2007, up from 8.1% in 200613. The ratio for members of Rx&D was 8.9%, up from 8.5% in the previous year. R&D-to-sales ratios for all patentees and for Rx&D members had declined in recent years, after rising from 1988 to the mid-1990's. As of 2007, the ratio for all patentees has remained below 10% for seven consecutive years, while the ratio for Rx&D members has been less than 10% for the last five years.
The Global Context
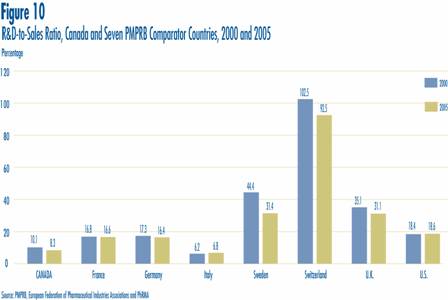
Figure 10 compares Canadian R&D-to-sales ratios to those in the PMPRB's seven comparator countries for the years 2000 and 2005. As noted in Figure 10, Canada's ratio stood at 10.1% in 2000. Only Italy, at 6.2%, had a lower ratio in that year. Switzerland had the highest ratio at 102.5%, followed by Sweden at 44.4%. France, Germany and the U.S. were in the 16-18% range, while the U.K. was more than double (35.1%). A very similar pattern emerges in the investment-to-sales ratios for 2005. Italy (6.8%) remained at the bottom of the range, with Canada second lowest at 8.3%. Ratios in all other comparator countries remained well above Canada's ratio.
Analytical Studies of Pharmaceutical Trends
National Prescription Drug Utilization Information System
The National Prescription Drug Utilization Information System (NPDUIS) provides critical analyses of price, utilization and cost trends in Canada. The Canadian Institute for Health Information (CIHI) and the PMPRB are partners in this initiative.
The NPDUIS initiative involves two major elements:
- development of a database incorporating data on individual claims made against public drug plans; and
- production of analytical reports using information in this database.
CIHI is responsible for the first of these elements while the PMPRB (as requested by the Minister of Health under section 90 of the Patent Act) is principally responsible for the second. A steering committee, comprised of representatives of participating public drug plans (all jurisdictions except Quebec) and Health Canada, advises the PMPRB on its research agenda and individual studies.
A major new NPDUIS periodical report was inaugurated in 2007. The New Drug Pipeline Monitor (NDPM) summarizes information on new drugs that are expected to be launched in Canada within the next two to five years and could have a significant impact on the expenditures of public drug plan expenditures. The first report was released in June 2007.
As well, a report entitled Budget Impact Analysis Guidelines was released in May 2007. This report provides a best-practices framework for predicting the likely financial impact on a drug plan of listing a new medicine.
At the end of March 2008, several new NPDUIS reports were being prepared for release in the near future.
- The 2008 edition of the Pharmaceutical Trends Overview Report (PTOR) will update the PMPRB's previous analyses of expenditure trends among public drug plans in Canada.
- A second issue of the NDPM report will update the set of “pipeline” drugs, while providing a special review of oncology drugs in development.
- A new study will examine the impact of long-term demographic change on public drug plans in Canada.
- A new study will analyze recent trends in reimbursement of dispensing fees and other costs incurred at the retail level.
- Obtaining meaningful measures of treatment volumes is a critical step in cost-driver and utilization analysis. A new study will critically evaluate methodological options for doing this.
In addition, best-practices guidelines are being developed for the forecasting of drug plan expenditures at the level of therapeutic class. It is expected these guidelines will be published by the end of 2008.
All studies conducted under the NPDUIS are available on the PMPRB Web site, as is a list of ongoing projects.
Monitoring and Reporting of Non-Patented Prescription Drug Prices
In October 2005, the federal/provincial/territorial Ministers of Health announced the endorsement of the PMPRB to monitor and report on non-patented prescription drug prices (NPPDP). In November 2005, the PMPRB received direction from the federal Minister of Health, on behalf of himself and his colleagues, to undertake this monitoring and reporting. To-date, four reports have been released:
- Canadian and Foreign Price Trends (July 2006), which examined domestic and international price and sales trends of non-patented prescription drugs;
- Trends in Canadian Sales and Market Structure (October 2006), which analyzed annual growth rates in sales, sources of growth, market shares, sales concentration, and international price comparisons by level of concentration;
- Market for New Off-Patent Drugs (June 2007), which examined market-entry and pricing behaviour among drugs that had recently gone off-patent; and
- Non-Patented Single-Source Drugs in Canada (November 2007), which examined price trends among non-patented drugs with only a single Canadian supplier.
Two new NPPDP reports are being prepared for release in 2008. These will update the first two reports listed above, applying several methodological refinements, and focusing on generic drugs.
As of April 2008, NPPDP studies are conducted under the umbrella of the NPDUIS.
