ARCHIVED - Health Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
2006-2007
Departmental Performance Report
Health Canada
The Honourable Tony Clement
Minister of Health
Table of contents
- Minister's Message
- Management Representation Statement
- Summary Information
- Overall Departmental Performance
- Summarizing Health Canada's Performance -- Our Medium-Term Corporate Priorities
- Our strategic Outcomes and Program Activities
Section II: Analysis of Performance by Strategic Outcome
- Health Canada's Program Activity Architecture (PAA)
- Strategic Outcome #1: Strengthened Knowledge Base to Address Health and Health Care Priorities
- Strategic Outcome #2: Access to Safe and Effective Health Products and Food and Information for Healthy Choices
- Strategic Outcome #3: Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments
- Strategic Outcome #4: Better Health Outcomes and Reduction of Health Inequalities between First Nations and Inuit and Other Canadians
Section III: Supplementary Information
- Organizational Chart
- Table 1: Comparison of Planned to Actual Spending (incl FTEs)
- Table 2: Resources by Program Activity
- Table 3: Voted and Statutory Items
- Table 4: Services Received without Charge
- Table 5: Sources of Respendable and Non-Respendable Revenue
- Table 6: Resource Requirements by Branch
- Table 7a: User Fees Act
- Table 7b: Policy on Service Standards for External Fees
- Table 8: Major Regulatory Initiatives
- Table 9: Details on Project Spending
- Table 10a: Summary of Transfer Payments by Program Activity
- Table 10b: Details on Transfer Payment Programs (TPPs)
- Table 11: Foundations -- Conditional Grants
- Table 12: Financial Statements
- Table 13: Responses to Parliamentary Committees, and Audits and Evaluations
- Table 14: Sustainable Development
- Table 15: Procurement and Contracting
- Table 16: Service Improvements
- Table 17: Horizontal Initiatives
- Table 18: Travel Policies
- Table 19: Storage Tanks
Section IV: Other Items of Interest
- Health Canada's Regional Structure and Operations
- Supporting Health Canada's Programs and Services
- The Health Canada Sustainable Development Strategy
- Advancing the Science Agenda
Section 1: Overview
Minister's Message
 The Departmental Performance Report (DPR) is a cornerstone of government accountability to Parliament and to Canadians. It is a public record of accomplishments and lessons learned. As Minister of Health, I am particularly pleased to share this DPR because it covers our
first full year as a Government. It outlines progress that we made to fulfill our commitments to Canadians in the Speech from the Throne, the 2006-2007 Report on Plans and Priorities, the 2006 and 2007 budgets and throughout the year. It also demonstrates sound governance and improved accountability in the delivery of our mandate.
The Departmental Performance Report (DPR) is a cornerstone of government accountability to Parliament and to Canadians. It is a public record of accomplishments and lessons learned. As Minister of Health, I am particularly pleased to share this DPR because it covers our
first full year as a Government. It outlines progress that we made to fulfill our commitments to Canadians in the Speech from the Throne, the 2006-2007 Report on Plans and Priorities, the 2006 and 2007 budgets and throughout the year. It also demonstrates sound governance and improved accountability in the delivery of our mandate.
In the 2006 Speech from the Throne, our Government specifically committed to negotiate Patient Wait Times Guarantees with the provinces as one of just five key priorities. I worked closely with my counterparts in the provincial and territorial governments and with people across Canada's health system to make these guarantees a reality.
Our government began support of four pilot projects that are building knowledge and identifying best practicesrelated to Patient Wait Times Guarantees and the reduction and better management of wait times. By April 2007, each provincial and territorial government agreed to establish a Patient Wait Times Guarantee by 2010, in at least one of the following priority clinical areas: cancer radiation, cataract surgery, hip and knee replacement, cardiac care, diagnostic imaging and primary health care. Our additional Budget 2007 funding will help to accelerate the kinds of wait times results that Canadians want and deserve.
As Minister, I have underlined the priority that I attach to ensuring that this country is well prepared to deal with the threat of avian flu and potential emergence of pandemic influenza. I know how important it is for plans to be in place, for the necessary supplies to be ready and for international collaboration and information sharing to be established. Over the past year, I have overseen actions that mean Canada is much better able to deal effectively with these threats that could arise at any time.
Cancer remains a serious threat to Canadians and it is one that we addressed early in our mandate. This year alone, some 160,000 Canadians will be diagnosed with cancer; it will take the lives of over 70,000. Our Government heard the call of more than 700 cancer survivors and experts to pool expertise and knowledge in order to reduce the toll of cancer in our country. We answered that call in Budget 2006 with $260 million over five years for the Canadian Strategy for Cancer Control. My Department led the federal government work to create the Canadian Partnership Against Cancer, which the Prime Minister announced in November 2006. The Partnership is already guiding the flow of our Budget money in a better-coordinated approach to achieve an estimated reduction of 1.2 million cases of cancer over the next 30 years, and to prevent 423,000 cancer deaths.
Many of this Department's actions have been part of government-wide commitments over the past year. An excellent example of a shared commitment to results is the Health Canada role in our Government's Chemicals Management Plan, which the Prime Minister announced in December 2006. Under the Plan, this Department is already beginning to assess those chemicals that entered Canadian use between 1984 and 1986 for their potential threats to human health. Just as we did for more recently introduced chemicals, Health Canada scientists are building an evidence base for sound scientific decisions on the future use of these chemicals. While this DPR provides details on our many steps forward to help improve the health of Canadians, I want to end this message by mentioning our launch of a revised version of Canada's Food Guide to Healthy Eating in February 2007. The health of Canadians is influenced by an extremely diverse range of factors. When we as individuals make choices such as a healthy diet and regular exercise, we make choices with clear and proven benefits. In a time with so many conflicting health claims and so many questions about health, it is important to be able to turn to reputable, sound sources - and Health Canada continues to consolidate its reputation as that kind of source for Canadians.
Canada's Food Guide and our many other information resources are tools that help Canadians make informed choices. They demonstrate that not only does our Government work with partners in other governmentsand across the health system to make that system work well, we want individuals to have the power and tools to make their own choices for better health for themselves and their families.
While we are proud of the results that we have generated in our first full year as a Government, we know there is much more work to accomplish in the years ahead.
The Honourable Tony Clement
Minister of Health Government of Canada
Management Respresentation Statement
I submit for tabling in Parliament, the 2006-2007 Departmental Performance Report for Health Canada.
This document has been prepared based on the reporting principles contained in the Guide for the Preparation of Part III of the 2006-2007 Estimates: Reports on Plans and Priorities and Departmental Performance Reports:
- It adheres to the specific reporting requirements outlined in the Treasury Board Secretariat guidance;
- It is based on the Department's Strategic Outcomes and Program Activity Architecture that were approved by the Treasury Board;
- It presents consistent, comprehensive, balanced and reliable information;
- It provides a basis of accountability for the results achieved with the resources and authorities entrusted to it; and
- It reports finances based on approved numbers from the Estimates and the Public Accounts of Canada.
Morris Rosenberg
Deputy Minister
Summary Information
About Health Canada
Health Canada develops, implements and enforces regulations, legislation, policies, programs, services and initiatives and works with other federal partners, the provinces and territories to maintain and improve the overall health of Canadians. As administrator of the Canada Health Act, we ensure that the principles of Canada's universal health care are respected, allowing Canadians to be confident in the services they receive from the public health care system. The Minister of Health is also responsible for the direct administration of another 18 statutes including the Food and Drugs Act, the Pest Control Products Act and the Controlled Drugs and Substances Act1.
We provide policy leadership and portfolio coordination among our partners in the Government of Canada's Health Portfolio, each of which produces its own Report on Plans and Priorities, namely:
- Public Health Agency of Canada;
- Canadian Institutes of Health Research;
- Hazardous Materials Information Review Commission;
- Patented Medicine Prices Review Board;
- Assisted Human Reproduction Agency of Canada.
Our Vision
Health Canada is committed to improve the lives of all people in Canada and to make Canada's population among the healthiest in the world as measured by longevity, lifestyle and effective use of the public health care system.
Our Mission
Health Canada is the federal department that helps the people of Canada maintain and improve their health.
Our Objectives
By working with others in a manner that fosters the trust of Canadians, Health Canada strives to:
- prevent and reduce risks to individual health and the overall environment and enhance the sustainability, innovation and integration of the health system;
- promote healthier lifestyles through sustained health protection and regulations;
- ensure high quality health services that are efficient and accessible;
integrate renewal of the health care system with longer term plans in the areas of prevention, health promotion and protection; - reduce health inequalities in Canadian society; and
- provide health information to help Canadians make informed decisions.
Our Roles
Health Canada employees play key roles in promoting, protecting and improving the health of Canadians - roles that assist other stakeholders working towards the same goals.
Health Canada operates in all regions of Canada as indicated on the accompanying map.
Health Canada at Work Across the Country
Innovators
As a science-based department, Health Canada employees are innovators, providing leading-edge science, sound policy research, and effective program and service development. Keeping abreast of global developments on diseases enabled Health Canada to play a leading role in Canada's response to the SARS, BSE and West Nile virus outbreaks.
Knowledge Brokers
Through research, risk assessments and surveillance, Health Canada provides knowledge to Canadians and others working in the health care field to enable them to make sound choices to protect health. The Department also monitors and researches health threats from environmental factors such as toxic substances, air and water pollution, climate change and other threats. This work fosters sound decision making and policy development at all levels to help reduce health risks.
Enablers
In all program areas, Health Canada brings stakeholders together, as well as provides information, research and education. The work of Health Canada enables Canadians to be up-to-date and informed about issues that can impact their health.
Trustees / Stewards
Health Canada, through administration of the Canada Health Act, aims to ensure that all eligible residents of Canada have reasonable access to medically necessary insured services. The Department's broad regulatory responsibilities to protect Canadians and promote health and safety range from prescription drugs and vaccines to toxic substances, from cardiac pacemakers to natural health products and food, from consumer goods to pesticides.
Proponents of Transparency
All work at Health Canada, from assessment of products under the Canadian Environmental Protection Act to regulation and approval of thousands of products is conducted transparently. Health Canada has committed to be accountable in delivering results to Canadians. The public had an opportunity to be involved in consultations on major regulatory initiatives such as the Pest Control Products Act and will continue to be consulted in other areas as part of the Department's consultations framework.
1 For more information on legislative acts, please visit the Department of Justice website
Overall Departmental Performance
Financial Resources (millions of dollars):
|
Planned Spending
|
Authorities*
|
Actual Spending**
|
|
3011.1
|
3090.1
|
2997.5
|
Human Resources (FTEs) :
|
Planned
|
Actual
|
Difference
|
|
8711
|
8686
|
25
|
* The increase from Planned Spending to Authorities is due mainly to new program initiatives and sustainability funding which is received through Supplementary Estimates.
** The difference between Authorities and Actual Spending is mainly the result of lapses in the TB Special Purpose and Frozen Allotments.
Our Operating Environment and Context
Health is a fundamental priority of the Government and Health Canada is the focal point for much of the federalhealth agenda. During 2006-2007, as in previous years, Health Canada worked closely with our Health Portfolio partners. We also collaborated with other federal departments on issues of shared responsibility such as environmental health, agriculture and improvements to regulatory approaches.
Health Canada continued to consult with a wide spectrum of partners: provincial and territorial governments, First Nations and Inuit organizations and communities, professional associations, consumer groups, universitiesand research institutes, international organizations and volunteers.
The Department used a mix of policy development and program delivery activities to carry out its responsibilities. Health Canada's grants and contributions programs funded partners in the health sector and at the community level to pursue shared goals, such as health system modernization and improved health outcomes for First Nations and Inuit. To support greater control over their health services, Health Canada also continued to transfer funding and responsibilities to First Nations and Inuit for the provision of many programs and services.
Health Canada's operating context in 2006-2007 evolved largely as projected in the Report on Plans and Priorities (RPP). The Government established key health commitments such as Patient Wait Times Guarantees; action to ensure Canada's preparedness for pandemic influenza and implementation of the Canadian Strategy for Cancer Control. We recognized the resource pressures affecting many of our activities and the need to modernize some of our core work such as regulation of health products and food.
Summarizing Health Canada's Performance - Our Medium Term Corporate Priorities and Key Areas of Focus
Health Canada continued to address four medium-term corporate priorities established in 2004 and further articulated and revised in the 2006-2007 RPP. These are based on the Department's vision, mission, and mandate, as well as on government directions and commitments, including First Ministers' Agreements. The priorities
integrated activities across all strategic outcomes.
Working with others to strengthen the efficiency and effectiveness of the publicly-funded health care system
(Including the key area of focus for 2006-2007: Develop the building blocks for establishing a Patient Wait Times Guarantee)
In its 2006 Speech from the Throne, the Government established its commitment to negotiate Patient Wait Times Guarantees as one of five key priorities. This led to discussions on research, and knowledge exchanges on wait times initiatives with governments of the provinces and territories. For example, our department began support of four pilot projects to help advance knowledge and best practices related to Patient Wait Times Guarantees and the reduction and better management of wait times. Pilot projects addressed diabetes and prenatalcare for First Nations communities. An additional pilot project focussed on wait times for children in need of surgery.
By April 2007, the government of each province and territory had committed to establishing a Patient Wait Times Guarantee by 2010, in one or more of: cancer radiation, cataract surgery, hip and knee replacement, cardiac care, diagnostic imaging and primary health care. Those governments also agreed to launch at least one pilot project to test guarantees and how they can best be implemented. The Quebec National Assembly passed legislation that establishes a framework in Quebec to guarantee access to hip and knee replacement and to cataract surgery.
That work will be accelerated with the Budget 2007 commitment of $1billion from 2006-2007 expenditures to fund a Patient Wait Times Guarantee Trust. The Trust will make payments over three years to support actions by the provinces and territories. Other funds were committed in that budget for 2007-2008 and for subsequent investments in electronic health information systems and to support provincial and territorial Patient Wait Times Guarantee pilot projects.
Projects under the Primary Health Care Transition Fund came to an end as scheduled during the year and we worked with partners to make the results of those projects widely known. To help solve health human resource challenges we focused on improvements to health care workplaces to encourage professionals to stay in those settings. The Department also supported provinces and territories as they created opportunities for internationally educated health professionals to earn Canadian credentials. In November 2006 the Government signed a Memorandum of Understanding with the Province of British Columbia and the British Columbia First Nations Leadership Council. This MOU committed the parties to building a tripartite relationship for improving the health of BC First Nations, and led to the signing of the Tripartite First Nations Health Plan for British Columbia in June 2007.
Reducing the risks to the health of the people of Canada
(Including the key area of focus for 2006-2007: Advance efforts to prepare for a global pandemic outbreak)
Risks to health take many forms and Health Canada has many ongoing regulatory responsibilities that seek to reduce those risks. The Department's direct activities in fields such as the safety of health products, food, consumer products and pest management products, as well as support for the work of other levels of government
in such areas as drinking water safety all contribute to the health of Canadians.
Health Canada is a participant in the government-wide effort led by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency to ensure that Canada is well prepared to deal with the threat of avian flu and potential emergence of pandemic influenza. For example, we improved our regulatory system in order to respond quickly to submissions for new vaccines that may be needed and to track their results once they are in use. Provincial and territorial health care systems are responsible for meeting the needs of most Canadians in a pandemic influenza, with PHAC responsible for some cases such as quarantines involving passengers and crews of aircraft, ships and trains. Health Canada, in collaboration with PHAC, is working with First Nations, and provinces and territories to develop integrated and coordinated pandemic response plans for First Nations at the community level. We also worked closely with health officials in other countries to improve information sharing and collaborative action on avian flu and pandemic influenza.
Health Canada's contributions formed a central element of the Government's launch of the Chemicals Management Plan in December 2006. That Plan will regulate chemicals that are harmful to human health or the environment and is part of the Government's comprehensive Environmental Agenda. It will expand the rigorous assessment of chemicals for health risks to include those introduced between 1984 and 1986. This will complement the work we completed in 2006-2007 involving our categorization of the 23,000 substances already placed on the Domestic Substances List for health implications.
While not specifically identified in our RPP, in October 2006, we released the Blueprint for Renewal: Transforming Canada's Approach to Regulating Health Products and Food for consultation. The Blueprint articulates our vision and plan over the coming years to modernize a regulatory system for health products and food that has essentially been in place since 1953. The Blueprint targets creation of a progressive licensing framework that will evaluate and monitor the safety, quality and effectiveness of health products, such as pharmaceuticals, throughout the years they are in use in Canada. This would replace the current focus on a company's initial request to gain approval for Canadian use of a product. The Blueprint is meant to ensure that Canadian legislation, regulations and practices keep pace with advancing science and technology, existing and emerging public health challenges, consumer expectations in terms of safety, the need for transparency, faster drug approvals and international developments.
That process of modernization will be similar in many respects to the effort we completed during 2006-2007 to implement the Pest Control Products Act. The new Act significantly increases transparency, enables greater public participation, expedites the registration of lower risk products and includes a new process to better protect human health and the environment. Communication and stakeholder engagement ensured that Canadians, especially businesses and major users of pest control products, were properly informed about the Act, and enabled us to gain feedback on opportunities to improve our services.
Contributing to the improvement of the health of Canadians
(Including the key area of focus for 2006-2007: Implement the Canadian Strategy for Cancer Control)
Health Canada has many roles that contribute to the improvement of the health of Canadians. For example, our launch of the revised Canada's Food Guide to Healthy Eating in February 2007 and the number of requests for printed copies as well as visits to the online version showed the demand for this kind of resource to promote eating choices that meet nutrient needs, promote health and minimize the risk of nutrition-related chronic disease. The culturally-specific Food Guide for First Nations, Inuit and Métis was also produced, tailored to reflect their traditions and food choices.
In Budget 2006, the Government allocated $260 million over five years to the Canadian Strategy for Cancer Control. While we continued cancer-related work, such as sun safety information for children to reduce the risk of skin cancer, our core role was to be the federal government's liaison with the Canadian Partnership Against Cancer announced by the Prime Minister in November 2006. The Partnership will collect, classify and distribute information on preventing, diagnosing and treating cancer, so that all health care providers have access to the best cancer care practices across Canada. It has the responsibility to implement a Strategy that experts have predicted could pre-empt 1.2 million cases of cancer and prevent 423,000 cancer deaths over the next 30 years.
We played a central role in developing options which led to the creation of the Mental Health Commission of Canada that was announced in Budget 2007. The Commission will undertake knowledge exchange, anti-stigma efforts and development of a National Strategy on Mental Health and Mental Illness. Health Canada's support included research into how gender could be integrated into federal mental health policy.
By far our largest category of expenditures as a department are our programs and services for addressing the health needs of First Nations and Inuit. These include primary health care services, home and community care, public health, and community programs aimed at children and youth, mental health and addictions, chronic and communicable diseases and environmental health, as well as the Non-Insured Health Benefits program that funds supplementary health benefits for all eligible First Nations and Inuit.
With increased funding to reflect growing First Nations and Inuit populations, the Department expanded programming in some areas. For maternal and child health, we implemented home visiting in 40 communities and launched a Healthy Pregnancy campaign to provide women with information to make healthy lifestyle choices before and during pregnancy. We provided funding to expand Aboriginal Head Start On-Reserve, to provide outreach in small communities, and improved early childhood development facilities.
Our Department obtained funding to provide mental health, emotional, and cultural supports to all eligible former students of Indian Residential Schools under the new IRS Settlement Agreement. Recognizing the much greater incidence of diabetes among Aboriginal people, First Nations diabetes-related services were the focus of two of four federal Patient Wait Times Guarantee pilot projects. We also enhanced diabetes prevention activities, diagnostic and complications screening, as well as care and treatment services. Community drinking water quality monitoring activities were also expanded.
Strengthening accountability to Parliament and the public
Health Canada has continued to implement a series of activities to respond to new and/or enhanced government-wide initiatives such as the Federal Accountability Act, the Public Service Modernization Act (PSMA) and the Management Accountability Framework (MAF). As well, to ensure greater accountability and transparency to Parliament and the public, the Department has developed an action plan and specifically committed to strengthening resource management and performance measurement / reporting in relation to its regulatory programs.
The Department has continued to move forward with the sustainable development (SD) initiative in all programs and activities. Health Canada's Sustainable Development Strategy III (SDS III 2004-2007): Becoming the Change We Wish to See is comprised of three themes: helping to create healthy social and physical environments; integrating sustainable development into departmental decision making and management processes; and minimizing the environmental and health effects of the Department's physical operations and activities.
Health Canada has successfully achieved various objectives and targets under each of the themes, including, for example, the drafting of an SD Policy Lens, which will undergo a pilot test in 2007, with the aim of improving SD considerations embedded in policies, plans and programs. We have also carried out the planning to create SDS IV, which will build on the lessons learned to date and set new directions for close alignment with government-wide SD efforts.
The 2006 Treasury Board Secretariat assessment of our Management Accountability Framework noted improvements in several areas, including IT management, citizen-focused services, effective procurement and extra-organizationalcontributions. It also noted the progress the Department has made in clarifying responsibilities and improving resource allocation to ensure accountability and a greater focus on priorities and results.
Our Chief Financial Officer Branch is leading a department-wide effort for improving management accountability and stewardship of resources. As part of the Financial Management Renewal Initiative led by the Office of the Comptroller General, Health Canada accelerated the development and implementation of its Financial Management and Control Framework, which, among other things, includes initiatives for enhancing budget management and assessing /ensuring readiness for audited departmental financial statements for 2008-2009.
We continued with the implementation of the Departmental Operational Planning (DOP) aimed at establishing clear linkages between priorities, planned activities, expected results, and proposed resource allocation. Enhancements to the DOP process have assisted management at all levels in focussing on priorities, identifying funding pressures, and facilitating reallocation of resources from lower to higher priorities.
As part of Treasury Board Management of Resource and Results Structure, the Department has established plans and commenced work to review and enhance baseline information for performance measurement for all areas, particularly regulatory programs. As well, the Department continues to focus on developing guidelines and tools to improve the quality and results-focus of evaluations, including the piloting of the "value for money" tool developed by Treasury Board Secretariat. The Department has also enhanced its effort for reviewing the performance measurement and evaluation strategies outlined in Treasury Board submissions and Memoranda to Cabinet.
The Department continued to integrate more rigorous management practices into its operations, including enhancement of the management of contracts, grants and contributions by ensuring that solid governance structures and administrative processes are in place. As well, the Department implemented Phase 1 of an automated Contract Requisition and Reporting System that affords more effective controls over contract administration.
Our strategic Outcomes and Program Activities
Strategic Outcome - Strengthened Knowledge Base to Address Health and Health Care Priorities
| (MILLIONS OF DOLLARS) |
Planned Spending
|
Authorities
|
Actual Spending
|
|
|
288.4
|
312.6
|
290.4
|
||
|
Program Activity
|
Expected Results
|
Performance Status
|
||
| Health Policy, Planning and Information |
Goals and objectives identified for specific strategies and initiatives. Knowledge development and transfer of specific health policy issues. |
Satisfactorily Met:
Challenge: |
||
Access to Safe and Effective Health Products and Food and Information for Healthy Choices
| (MILLIONS OF DOLLARS) |
Planned Spending
|
Authorities
|
Actual Spending
|
|
|
262.1
|
278.2
|
262.3
|
||
|
Program Activity
|
Expected Results
|
Performance Status
|
||
| Health Products and Foods | Access to safe and effective health products and food and information for healthy choices. |
Satisfactorily Met:
Challenges: |
||
Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments - A
| (MILLIONS OF DOLLARS) |
Planned Spending
|
Authorities
|
Actual Spending
|
|
|
289.9
|
305.3
|
294.1
|
||
|
Program Activity
|
Expected Results
|
Performance Status
|
||
| Healthy Environments and Consumer Safety |
Improved scientific knowledge and capacity within the Canadian scientific community and international collaboration Availability and Canada-wide adoption of measures to control the risks to human health posed by environmental contaminants. Reduced risk of death and injury from exposure to hazardous products and substances associated with solar UV radiation. Reduced health and safety risks associated with tobacco consumption and the abuse of drugs, alcohol |
Satisfactorily Met:
Challenges: |
||
Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments - B
| (MILLIONS OF DOLLARS) |
Planned Spending
|
Authorities
|
Actual Spending
|
|
|
51.6
|
68.0
|
62.7
|
||
|
Program Activity
|
Expected Results
|
Performance Status
|
||
| Pest Control Product Regulation |
Access to safer pesticides. Transparency of pesticide regulation. Improved regulatory efficiencies and cost effectiveness. Informed public and stakeholders. |
Satisfactorily Met:
Challenges: |
||
Better Health Outcomes and Reduction of Health Inequalities between First Nations and Inuit and Other Canadians
| (MILLIONS OF DOLLARS) |
Planned Spending
|
Authorities
|
Actual Spending
|
|
|
2,119.1
|
2,126.0
|
2,088.0
|
||
|
Program Activity
|
Expected Results
|
Performance Status
|
||
| First Nations and Inuit Health | Improve health outcomes, by ensuring the availability of, and access to, quality health services, and, supporting greater control of the health system by First Nations and Inuit. |
Satisfactorily Met:
Challenges: |
||
Section II Analysis of Performance by Strategic Outcome
Health Canada's Program Activity Architecture (PAA)
This section reports on our results in detail based on our PAA, which links budgets to expenditures and, in turn, to performance.
Planned and Actual Spending by Strategic Outcome, Program Activity and Sub-Activity
(millions of dollars)
| Program Activity | Planned Spending | Authorities | Actual Spending | Program Sub-Activities |
|---|---|---|---|---|
|
Strategic Outcome #1 |
||||
| Health Policy, Planning and Information | 288.4 | 312.6 | 290.4 | |
| 158.1 | 162.9 | 147.7 | Health Care Policy | |
| 6.6 | 6.6 | 4.8 | Intergovernmental Affairs | |
| 16.6 | 19.5 | 12.9 | Strategic Health Policy | |
| 25.0 | 38.4 | 37.9 | International Affairs | |
| 5.4 | 5.7 | 5.4 | Women's Health | |
| 34.8 | 35.4 | 32.2 | Applied Research, Dissemination and Accountability | |
| 1.0 | 0.9 | 1.9 | Nursing | |
| 40.9 | 43.2 | 47.6 |
Official Language Minority Community Development |
|
| Strategic Outcome #2 Access to Safe and Effective Health Products and Food and Information for Healthy Choices |
||||
| Health Products and Food | 262.1 | 278.2 | 262.3 | |
| 125.8 | 133.6 | 125.9 | Pre-market Regulatory Evaluation and Process Improvement | |
| 13.1 | 13.9 | 13.1 | Information, Education and Outreach on Health Products, Food and Nutrition | |
| 104.9 | 111.2 | 104.9 | Monitoring Safety and Therapeutic Effectiveness and Risk Management | |
| 18.3 | 19.5 | 18.4 | Transparency, Public Accountability and Stakeholder Relationships | |
|
Strategic Outcome #3(a) |
||||
| Healthy Environments and Consumer Safety | 289.9 | 305.3 | 294.1 | |
| 29.9 | 40.5 | 40.5 | Workplace Health and Public Safety | |
| 83.5 | 86.1 | 85.0 | Safe Environments | |
| 31.3 | 33.1 | 31.1 | Product Safety | |
| 65.7 | 66.4 | 60.8 | Tobacco Control | |
| 79.5 | 79.2 | 76.7 | Drug Strategy and Controlled Substances | |
|
Strategic Outcome #3(b) |
||||
| Pest Control Product Regulation | 51.6 | 68.0 | 62.7 | |
| 25.6 | 33.6 | 27.5 | New Pest Control Product Registration and Decision making | |
| 9.8 | 12.8 | 12.1 | Registered Pest Control Product Evaluation and | |
| 7.6 | 10.0 | 11.2 | Decision making Compliance | |
| 2.6 | 3.3 | 4.1 | Pesticide Risk Reduction | |
| 6.0 | 8.3 | 7.8 | Regulatory Improvement | |
|
Strategic Outcome #4 |
||||
| First Nations and Inuit Health | 2,119.1 | 2,126.0 | 2,088.0 | |
| 292.9 | 326.2 | 290.7 | First Nations and Inuit Community Health Programs | |
| 76.5 | 71.9 | 69.6 | First Nations and Inuit Health Protection | |
| 247.0 | 270.5 | 289.0 | First Nations and Inuit Primary Health Care | |
| 966.3 | 1,018.7 | 996.4 | Non-Insured Health Benefits (NIHB) | |
| 536.4 | 438.7 | 442.3 | Governance and Infrastructure Support to First Nations and Inuit Health System | |
Note: The DPR highlights results achieved for key initiatives and PAA sub-activities outlined in the 2006-2007 Report on Plans and Priorities.
Strategic Outcome #1 - Strengthened Knowledge Base to Address Health and Health Care Priorities
Program Activity Name: Health Policy, Planning and Information
Expected Results:
- Goals and objectives identified for specific strategies and initiatives
- Knowledge development and transfer of specific health policy issues
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
| 288.4 | 312.6 | 290.4 |
Human Resources (FTEs):
| Planned | Actual | Difference |
|---|---|---|
| 627 | 606 | 21 |
The objective of this program activity is to provide policy advice and support to the Minister in making decisions to protect and improve the health of Canadians. Health Canada develops policies and builds and maintains linkages with provinces, territories, and other partners and stakeholders to support health care system reform. We administer the Canada Health Act and facilitate access to health care services for official language minority communities. We work with international organizations, and bilaterally with key countries, to advance a global health agenda. Grants and contributions accounted for approximately 68 percent of spending under this program activity, demonstrating our commitment to achieving results in collaboration with partners in the health system.
Grants Explanation of the above financial information:
Variances between planned spending versus total authorities are mainly due to:
- funding for Federal Contaminated Sites Action Plan
- funding for the Canadian Partnership against Cancer
- contributions to the Government-Wide 2006-2007 $1 Billion Spending Restraint Exercise
- funding from Management Reserve - Litigation Management.
Actual spending is $22.2 million lower than total authorities mainly due to:
- lapse in the Health Council special purpose allotment
- contributions to the Government-Wide 2006-2007 $1 Billion Spending Restraint Exercise
- year end adjustments of Department of Justice expenditures
- other operating lapses in various programs.
In the Report on Plans and Priorities (RPP), we identified eight priorities under this program activity, as well as two areas addressing the role of science and horizontal linkages.
Partner in Health Reform
Canadians expect to have accessible, high quality health care services throughout their lives. To this end, the Department supported development of Patient Wait Times Guarantees (PWTGs), the Health Council of Canada and the Primary Health Care Transition Fund (PHCTF). The Department also continued to support initiatives in health human resources, home care, palliative care and access to health care for people living in official language minority communities.
One of the Government's five priorities was to negotiate PWTGs with provincial and territorial governments. All provinces and territories have now committed to establishing a PWTG by 2010 in one of the following priority clinical areas: cancer radiation, cataract surgery, hip and knee replacement, cardiac care, diagnostic imaging or primary health care. In addition, each province and territory has committed to undertaking at least one PWTG pilot project to test guarantees and inform their implementation. The Government of Quebec passed legislation to establish a framework to implement a guarantee of access to hip and knee replacement and cataract surgery.
Our Department also developed four PWTG pilot projects to help advance best practices and the reduction and better management of wait times. Two projects focus on diabetes and prenatal care in selected First Nations communities and are administered by Health Canada's First Nations and Inuit Health Branch. A third project addresses wait times for diabetic foot ulcer care for First Nations communities in Manitoba and is being administered by Saint Elizabeth Health Care. A fourth project in national paediatric surgical wait times is led by the Paediatric Surgical Chiefs of Canada and the Hospital for Sick Children in Toronto.
Health Canada also provided funding to the Canadian Institutes of Health Research to support research regarding the relationship between wait times and health and improving access to appropriate health services. In February 2007, the Minister of Health and his Saskatchewan counterpart co-hosted a Conference on Timely Access to Health Care that showcased provincial successes and innovations. Further, Health Canada released the report, Healthy Canadians: A Federal Report on Comparable Health Indicators 2006, which provides a snapshot of the health status of Canadians and the performance of the health care system. The report also responds to First Ministers' commitments to greater transparency and accountability in the health care system.
Following the 2004 First Ministers' agreement to provide first-dollar coverage for certain home care services, we provided policy advice within the Department and to federal partners concerning access by First Nations, Inuit and veterans to home care services at the expected levels. In March 2007, we hosted a forum with provincial and territorial stakeholders to look at opportunities for integration between home and primary health care. As well, our Department is exploring ways to work with the Canadian Home Care Association to advance home care integration models across Canada.
Health Canada continued to support the work of the Health Council of Canada, which has a mandate to monitor and report on implementation of the 2003 and 2004 Health Accords and to report annually on health status and health outcomes of Canadians.
In response to a First Ministers' 2000 commitment, the PHCTF was launched. Between 2001 and 2006, this $800 million federal investment supported the efforts of provinces, territories and stakeholders to reform the primary health care system. With the end of the PHCTF, our focus turned to applying lessons learned across Fund projects to support ongoing primary health care reform activities.
Key PHCTF dissemination activities included synthesis papers highlighting results on chronic disease management and collaborative care; a national conference in February 2007; a Best Practices Network event on responding to community needs; and fact sheets and a database on the results of each PHCTF initiative.
We supported programs and services to improve access to health care for people in official language minority communities, consistent with the Official Languages Act. Two contribution agreements were launched to provide primary health care services within English and French-speaking minority communities.
Health Canada hosted a national forum on Palliative and End-of-Life Care in March 2007. The purpose was to support exchange of best practices across Canada, as well as to celebrate five stakeholder groups for palliative and end-of-life care initiatives supported by Health Canada from 2002 to 2007. We also provided funding for development of a web-based research centre at the Canadian Virtual Hospice. This will ensure that the growing community of palliative care researchers have the necessary tools to provide the scientific basis for improving care.
- Conference on Timely Access to Health Care
- Healthy Canadians: A Federal Report on Comparable Health Indicators 2006
- Primary Health Care Transition Fund
Hepatitis C
The pre-1986/post-1990 Hepatitis C Settlement Agreement was completed in December 2006. Preparations were initiated for the compensation process, subject to a decision expected in 2007 by the courts that have been involved in this process. This should lead to the first payments beginning in 2007-2008.
Pandemic Influenza
Our Department played many roles to ensure Canada's readiness to deal with the potential emergence of pandemic influenza. These included discussions concerning innovative approaches and incentive mechanisms to stimulate research, development, and equitable global access to pandemic influenza vaccines, as well as pneumococcal vaccines. Internationally, as Chair of the Asia-Pacific Economic Cooperation (APEC) Health Task Force, Health Canada was playing a leadership role in coordinating regional response and planning for pandemic influenza and other emerging infectious diseases. We facilitated development of the APEC Action Plan on the Prevention and Response to Avian and Influenza Pandemics and followed up in 2007 with the first regional report on implementation of the plan.
Mental Health
In the RPP, we committed to work with partners to build the foundation of a national approach to mental health and mental illness. This led to the announcement of the Mental Health Commission of Canada in Budget 2007. The Commission will undertake activities in three areas: knowledge exchange, anti-stigma efforts, and a National Strategy on Mental Health and Mental Illness.
Pharmaceuticals Management
With respect to pharmaceuticals, Health Canada has a role in market approval, access, optimal prescribing and utilization, drug prices/expenditures and system cost, as well as the safety and effectiveness of drugs once on the market.
The Department's Blueprint for Renewal initiative is focused on modernizing the regulatory system for therapeutic products.
In collaboration with other departments, including Foreign Affairs and International Trade, Health Canada plays an important role in monitoring and research activities, policy development, and provision of integrated advice (reflecting health sector interests) on pharmaceuticals-related components of: international trade negotiations and treaties; transnational issues and files such as cross-border drug sales; patent policy; and cooperative research and knowledge exchange activities bilaterally and in international fora such as the Organization for Economic Cooperation and Development (OECD) and the World Health Organization (WHO).
In 2006, Health Ministers provided First Ministers with a National Pharmaceuticals Strategy Progress Report. The report highlighted achievements and set out next steps, with a focus on five priority areas: catastrophic drug coverage; expensive drugs for rare diseases; common national drug formulary; drug pricing and purchasing strategies; and real-world drug safety and effectiveness.
Health Canada is working to ensure that current work on the NPS complements pre-existing initiatives such as the Common Drug Review (CDR)7.
7The CDR is a single process for reviewing new drugs and providing listing recommendations to participating publicly-funded federal, provincial and territorial drug benefit plans. All jurisdictions are participating except Quebec. For more information, see http://www.cadth.ca/index.php/en/cdr and http://www.hc-sc.gc.ca/hcs-sss/pharma/mgmt-gest/cdr-emuc/index_e.html. Other joint initiatives that emerged prior to the NPS and which continue to progress include the Canadian Optimal Medication Prescribing and Utilization Service (COMPUS) and the National Prescription Drug Utilization System (NPDUIS).
Legislative Renewal and Regulatory Reform
Work continued to update the Food and Drugs Act, the Hazardous Products Act and the Radiation Emitting Devices Act. We focused on policy development for the collection, use and disclosure of health information and improved compliance and enforcement.
Health Canada improved its regulatory processes in response to the Smart Regulation Initiative and other government-wide policies. Triage and prioritization models were developed to ensure greater efficiencies while improving implementation of cost-benefit analysis, instrument choice and performance measurement.
Establishment of the Assisted Human Reproduction Agency of Canada and new regulations
The Assisted Human Reproduction Act (AHRA) seeks to protect and promote the health, safety, human dignity and rights of Canadians who are born of the use of reproductive technologies and to foster the application of ethical principles in relation to assisted human reproduction.
The Agency created under the Act is the Assisted Human Reproduction Agency of Canada. The Government announced the appointment of a Chairperson, President and eight additional members to its Board of Directors in December 2006. Health Canada continued research and consultations to develop the necessary regulatory framework for the AHRA. We reviewed feedback on section 8 (relating to consent) draft regulations which were subsequently published in Canada Gazette, Part II in June 2007 and will take effect on December 1, 2007. Health Canada continues to work on remaining regulations required by the AHRA.
Assisted Human Reproduction Agency of Canada
Health Human Resources
At the heart of any health care system are the people who deliver care – health human resources (HHR). Governments at the federal and provincial/territorial levels have recognized that it is critical to ensure the adequate supply, distribution and utilization of health human resources. In response to the 2003 and 2004 First Ministers' agreements on health, Health Canada worked to implement its responsibilities under the Pan-Canadian HHR Strategy which is being renewed for 2008-2013. The Strategy includes several initiatives: Pan-Canadian Health Human Resource Planning; Interprofessional Education for Collaborative Patient-Centred Practice; and Recruitment and Retention. In 2005, the Internationally Educated Health Professionals Initiative (IEHPI) was established to increase the supply of priority health care providers through assessment and integration of internationally educated health professionals. The IEHPI is a five-year $75 million initiative that is also part of the Pan-Canadian HHR Strategy. Multi-year contribution agreements are in place with most of the provinces and territories as well as innovative pan-Canadian projects covering seven health professions: medicine, nursing, pharmacy, occupational therapy, physiotherapy, medical laboratory technology and medical radiation technology.
As a result of its participation in the Federal Council's Ontario Information Technology Network, the Ontario Region was able to sign agreements with other federal departments to share infrastructure resources and to arrange interchange opportunities and assignments for staff. An interchange arrangement was helpful in quickly filling a vacancy in the Thunder Bay office.
We provided funding to key stakeholder-driven initiatives. For example, in response to the shortage of family physicians, the College of Family Physicians of Canada created Family Medicine Interest Groups that are using different tools to encourage more medical students to choose family medicine as a postgraduate specialty. We also supported the 2007 international conference of leaders in HHR research and policy to examine the medical work force as it concerns the evolution of health care delivery systems.
An innovative interprofessional program aimed at orienting IEHPs to the Canadian health care system was developed arrangement with representatives from provinces and territories as well as six health professions. A multi-media faculty development program for teachers of international medical graduates was developed and will be fully implemented in 2007-2008. Similarly, a curriculum for faculty members training internationally educated nurses was created and will be piloted in 2007-2008. Supported by Health Canada, Ontario's Access Centre for IEHPs was formally launched in December 2006, and has been providing IEHPs with referral, counselling and bridging services. As of April 2007, the Centre has supported close to 400 IEHPs to integrate into the health care system.Under Interprofessional Education for Collaborative Patient-Centred Practice, nine new learning projects were funded for $6.7 million, bringing the total to 20 projects and $20 million. The Canadian Interprofessional Health Collaborative received $775,000 to identify and share best practices in interprofessional education and collaborative practice, and to translate this knowledge so that it can be used to transform health care.
Health Human Resources Strategy
In addition to these priorities, we addressed two areas of importance across this program activity.
Role of Science
Innovative health sciences and technologies offer potential new ways to prevent, diagnose and treat thousands of conditions affecting Canadians. These include genetic technologies, stem cell research and nanotechnologies. They also present certain challenges, such as ensuring that intellectual property and patent rights concerning genetic inventions are compatible with appropriate patient access and that Canadians receive high quality genetic services.
In consultation with many partners in Canada, our Department worked closely with the OECD to develop OECD Guidelines on Quality Assurance in Molecular Genetic Testing that are to be published in 2007. The Guidelines are an important tool to promote safe, effective and appropriate use of genetic testing in Canada. During the electronic public consultation period on the Guidelines, Canada was recognized as having the highest stakeholder participation rate of any OECD member state.
Health Canada has played a key role in the Budget 2007 commitment to invest $30 million in the Rick Hansen Foundation's Spinal Cord Injury Translational Research Network. The goal is to accelerate the translation of research discoveries into practical benefits for Canadians with spinal cord injuries while generating savings in health and social services and accelerating scientific advances towards a cure.
Health Canada conducted extensive policy research on intellectual property and patents in medical genetics and stem cells. Through workshops and symposia, we have encouraged stakeholders to adopt OECD Licensing Guidelines for Genetic Inventions. These and other creative licensing strategies will help overcome patent-related barriers to research and improve patient access to innovative biotechnology products in a cost-effective manner.
Horizontal Linkages
Research activities focused on issues such as First Nations and Inuit health sustainability, public system health care comparative analysis, health innovation and healthy communities. We continued our examination of factors influencing sustainability of the health care system including the rapidly growing pharmaceutical sector and productivity in the health sector.
Consistent with a government-wide commitment, we addressed gender and diversity issues in areas such as mental health, cancer and clinical trials. We successfully negotiated for inclusion of health, gender and diversity considerations in the deliberations of the Working Group on Trafficking in Persons.
Health Care Policy
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
158.1
|
162.9
|
147.7
|
Health Canada provides policy leadership and advice on issues related to the health care system and its renewal, such as improving access to quality care, increasing the supply and improving the mix of health care professionals, and enhancing the accountability of the system to Canadians.
Policy leadership is also provided in health care delivery, particularly home care, continuing care, palliative care and primary health care, and also to issues such as the responsiveness of the health care system to aging, chronic disease management and e-health. Programs promote and facilitate effective and innovative planning, education, training, management, recruitment and retention of health human resources in Canada.
In addition to initiatives described previously under this strategic outcome, the Department provided policy leadership and coordination on other issues that are being addressed across the Health Portfolio. For example, we worked with many partners to identify how the Government of Canada could best address the needs for a coordinated approach to cancer. This supported the Government decision to create the Canadian Partnership Against Cancer (CPCC), a not-for-profit corporation. CPCC was established to implement the Canadian Strategy for Cancer Control, a five-year plan developed by more than 700 cancer survivors and experts. The CPCC has begun to take shape to serve as a clearing house for state-of-the-art information about preventing, diagnosing, and treating cancer. Its work is supported by the $260 million announced in the 2006 budget.
We also were assigned the responsibility of leading federal policy development in relation to autism spectrum disorders. This including guiding and supporting actions designed to expand understanding of autism among researchers and health professionals and to provide more information to families and others who deal with autism. Our efforts included close cooperation with partners such as the Public Health Agency of Canada and the Canadian Institutes of Health Research.
Intergovernmental
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
6.6
|
6.6
|
4.8
|
We continued to administer the Canada Health Act (CHA), which included investigations into potential cases of non-compliance and analysis of relevant emerging issues, such as patient charges for primary care in private facilities, possible extra-billing by physicians and charges for surgical services by private clinics. We continued to see the traditionally high level of provincial and territorial compliance, which we detail to Parliament and Canadians in the Canada Health Act Annual Report.
We provided strategic and tactical advice and support on the full range of intergovernmental health-related issues and activities, with emphasis on pandemic preparedness and Patient Wait Times Guarantees.
International
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
25.0
|
38.4
|
37.9
|
Health Canada continues to initiate, coordinate, and monitor departmental policies, strategies and activities that help promote Canadian priorities and values internationally. We collaborated with external health partners such as the WHO and the Pan American Health Organization (PAHO) on pandemic influenza preparedness, HIV/AIDS and global health security.
Health Canada coordinates the global engagement component of the Federal Initiative to Address HIV/AIDS in Canada, to ensure policy coherence of Canada's international HIV/AIDS activities. Notable achievements included a strong, coordinated and effective presence at the XVI International AIDS Conference in Toronto, August 13-18, 2006. Health Canada and the Public Health Agency of Canada signed a formal partnership agreement in August 2006 with the Joint United Nations Programme on HIV/AIDS (UNAIDS) to promote joint actions aimed at strengthening global response to the HIV and AIDS epidemic.
On August 15, 2006 in Toronto, the Health Ministers for Canada and France signed a Joint Declaration of Intent Regarding Cooperation Between the Department of Health of Canada and the Ministry of Health and Solidarity of the French Republic in the Field of Health for the Period 2006-2010. The Declaration is a framework arrangement that outlines mutual work the two countries plan to undertake in the next four years. Key areas are pandemic influenza preparedness, the strengthening of health care systems, HIV/AIDS, sexually transmitted infections, hepatitis B/C, tuberculosis and sexual and reproductive health. Other potential areas of cooperation could be on physical activity, cancer and mental health.
Assisted Human Reproduction Implementation Office
Health Canada provides policy analysis and advice towards the establishment of the Assisted HumanReproduction Agency of Canada as well as developing the regulatory framework required by the AHR Act.
Legislative Renewal
Legislative renewal activities concentrate on updating and strengthening health protection legislation to ensure that it is responsive to present and future social and technological realities.
Applied Research, Dissemination and Accountability
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
34.8
|
35.4
|
32.2
|
Health Canada helps to build the analytical foundation for health policy decision making, performance measurement and reporting. This includes conducting economic analysis of health policy issues, funding external policy research in priority areas, and running a policy research publications program, which includes publication of the Health Policy Research Bulletin. We develop, in collaboration with partners and stakeholders, federal policy on investments in Canada's health statistics system and coordinate departmental core data requirements with data providers.
Official Language Community Development
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
40.9
|
43.2
|
47.6
|
We provided leadership in responding to the health needs of official language minority communities by administering Health Canada's responsibilities under Section 41 of the Official Languages Act.
We supported programs and services to improve access to health care for people in official language minority communities, consistent with the Official Languages Act. Two contribution agreements were launched to provide primary health care services within English and French-speaking minority communities. A report analyzing the evolving health access concerns of Francophone minority communities was completed and presented to the Minister in February 2007 by the Consultative Committee for French-Speaking Minority Communities. Working with Statistics Canada and several other federal departments, we launched the 2006 Survey on the Vitality of Official Language Minorities to improve the Government's ability to measure their health-related challenges. The results of this Survey will be available from Statistics Canada in 2007-2008. We partnered with the Department of Canadian Heritage in launching a learning tool for development of status reports and action plans required under Part VII of the Official Languages Act and its accountability frameworks.
Strategic Outcome #2: Access to Safe and Effective Health Products and Food and Information for Healthy Choices
Program Activity Name: Health Products and Food
Expected Results:
- Access to safe and effective health products and food and information for healthy choices
| Performance Indicators | Results |
|---|---|
| Level of satisfaction of Canadians and health professionals with the information disseminated for healthy choices and informed decision making | This indicator is being revised. No current data exists. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
262.1
|
278.2
|
262.3
|
Human Resources (FTEs):
2,592| Planned | Actual | Difference |
|---|---|---|
|
2,592
|
2,563
|
29
|
Explanation of the above financial information:
- Variances between planned spending versus authorities are mainly due to:
- Funding from Management Reserve - Natural Health Products
- Funding from Management Reserve - Litigation Management
- Funding for Therapeutics Access Strategy.
The actual spending is $15.9 million lower than authorities mainly due to:
- Lapse of frozen allotment for Access to Medicines Regime
- Year end adjustments of Department of Justice expenditures
- Other operating lapses in various programs.
Health Products and Food is a regulatory program. Its objective is to evaluate and monitor the safety, quality and effectiveness of the thousands of drugs, vaccines, medical devices, natural health products and other therapeutic products available to Canadians, and nutritional quality of their food. We also review veterinary drugs sold in Canada as well as foods derived from animals treated with these drugs. We promote the health and well-being of Canadians by developing nutritional policies and standards such as Canada's Food Guide and providing information to the public in newsletters such as It's Your Health. The Government is committed to continually modernizing its legislative and regulatory frameworks to keep pace with advancing science and technology, existing and emerging public health challenges, consumer expectations in terms of safety, the need for transparency, faster drug approvals, international developments and other factors. Since 1953, responsibilities for health products and food safety have been primarily defined through the Food and Drugs Act.
Health Canada has identified challenges that must be met to ensure continued, timely access to safe and effective health products and a safe and nutritious food supply. These include but are not limited to: an outdated regulatory tool kit that is increasingly limited and inflexible in responding to today's environment; the regulatory system's incapacity to consider a product through its entire life cycle; and a regulatory system with insufficient resources for long-term efficiency and sustainability. The Office of the Auditor General came to similar conclusions when it reviewed this area for its November 2006 report. 8
Consequently, in October 2006, we released Blueprint for Renewal: Transforming Canada's Approach to Regulating Health Products and Food for consultation. This policy review document builds on progress made over the last few years to improve the regulatory system's efficiency, safety and transparency. The Blueprint reflects input from Canadians and commits us to improve information for decision making. It is supported by detailed action plans, setting the stage for consultations on and implementation of specific Blueprint initiatives in 2007-2008 and 2008-2009, including a new progressive licensing framework for pharmaceuticals and biologics. We will continue to report progress on these and longer-term initiatives through the Blueprint web-site and in reporting frameworks such as the DPR.
The Department addressed other areas that complement these directions as well as issues raised by the Auditor General. For example, we began work on a cost recovery framework and a comprehensive review of programs and resources. The cost recovery regime should be implemented in 2008-2009, updating a 10-year-old fee regime. This will support a long-term, stable and sustainable funding strategy for our regulatory programs. The assessment of all programs and activities will define the level of activities, performance and resources required to meet our regulatory and other responsibilities, based on the full cost of these activities.
Under this Health Products and Food program activity, are four sub-activities as defined in our Program Activity Architecture (PAA). Achievements under each of the sub-activities are outlined below.
8 OAG report: http://www.oagvg.gc.ca/domino/reports.nsf/html/20061108ce.html
Pre-market Regulatory Evaluation and Process Improvement
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
125.8
|
133.6
|
125.9
|
Improved timeliness, transparency and predictability of the regulatory process Improved timeliness, transparency
and predictability of the regulatory
process
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved timeliness, transparency and predictability of the regulatory process |
Percentage of overall workload in backlog and percentage of decisions issued within target for submission reviews of:
|
|
We continued to implement a long-term government commitment to improve the timeliness of the regulatory process for therapeutic products to ensure that Canadians have faster access to the safe drugs they need. This had many elements.
Improved Speed of Decision Making
Using funding received in Budget 2003 under the Therapeutics Access Strategy, a five-year, $190 million initiative, we improved our speed of decision making on new product reviews. This is demonstrated in the table above. In addition, we cleared the backlog of new biologic submissions by September 2006.
We made similar progress for medical device applications, which are assigned to classes based on their potential risk to humans. For example, bandages are Class I (low risk) devices, while pacemakers and HIV test kits are Class IV (highest risk). 11 Our processing of Class II applications was much better than the 51 percent performance in 2004. In the area of natural health products, we are making six times as many daily decisions as we did a year ago.
We continued to reduce decision times for veterinary drugs and set service standards. We reviewed 93 percent of data packages submitted prior to October 1, 2005, exceeding our target of 90 percent. We also completed several submission reviews that were not on our target list.
The ongoing challenge continues to be significant backlogs in our pre-market review of submissions for natural health products, veterinary drugs and food products. We have reduced backlogs at some stages of our work and are pursuing measures to process these applications more quickly and consistently in comparison with international performance targets.
The Blueprint for Renewal
Under the Blueprint described earlier, a progressive licensing framework for pharmaceuticals and biologics will facilitate access to drugs while continuing to monitor safety, efficacy, and quality throughout a drug's life cycle. The project achieved its early-stage objective and benefited from outreach to patient and consumer groups, industry, academia, health care professionals and provincial representatives. We expect to formulate a more detailed framework during 2007. 12
In 2001, Health Canada established a framework and regulations to oversee clinical trials that support applications for new drugs. At that time, we committed to assess the impact of the regulations and seek advice on improvements in three to five years. As part of the Blueprint initiative, we began a public e-consultation in June 2006. This led to a March 2007 workshop with stakeholders on specific possible improvements.
In addition to work under the Blueprint, we began the review of regulations and processes related to pre-market safety assessment and authorization of foods and food products. We developed a draft Guide for the Preparation of Submissions on Food Additives and an options paper for a modernized regulatory framework for food additives that is expected to be released soon for external consultation.
11 Classes of medical devices:
Class Class I| Class | Risk | Example |
|---|---|---|
| Class I | Lowest risk | Reusable surgical scalpel, bandages, culture media |
| Class II | Low risk | Contact lenses, epidural catheters, pregnancy test kits, surgical gloves |
| Class III | Moderate risk | Orthopedic implants, glucose monitors, dental implants, haemodialysis systems, diagnostic ultrasound systems |
| Class IV | High risk | HIV test kits, pacemakers, angioplasty catheters |
12 http://www.hc-sc.gc.ca/ahc-asc/branch-dirgen/hpfb-dgpsa/blueprint-plan/index_e.html
http://www.hc-sc.gc.ca/dhp-mps/homologation-licensing/develop/plan_e.html
Clinical Trials
In 2005, Health Canada received $170 million over five years to improve the safety of drugs, medical devices and other therapeutic products. As part of this initiative, we are strengthening the oversight of pharmaceutical clinical trials and investigational testing for medical devices conducted in Canada. New funding for 20062007 enabled us to add staff to handle significant increases in drug clinical trial applications, clinical trial Adverse Reaction Reports and safety reports. This has resulted in more timely responses and appropriate linkages with our pre-market review and post-market surveillance activities, although we recognize gaps that we must still address.
In addition, we increased efforts to make our internal processes as consistent and transparent as possible. As a result of posting the Clinical Trials e-manual on our website in 2006 we had fewer calls from clinical trial sponsors for information. We also discovered fewer deficiencies while screening clinical trials and investigational testing applications from clinical trial sponsors.
Regulatory Actions
We continued to develop regulatory frameworks. One seeks to minimize the potential health risks to Canadian recipients of human cells, tissues and organs through proposals for Safety of Human Cells, Tissues and Organs for Transplantation Regulations. Extensive consultations will continue in 2007-2008. We are at an earlier stage of consultations to guide a renewed regulatory framework for whole blood and blood components, typically used in transfusions, under the Food and Drugs Act.
Health Canada continued to lead a federal stewardship approach to manage risks and benefits of emerging biotechnologies and nanotechnologies products and services. This included work on horizontal policies, identification of barriers and implementation of strategies for supportive environments. An interdepartmental Biotechnology Regulatory ADM Steering Committee considered common biotechnology regulatory challenges among departments.
We released revised guidelines on the safety assessment of novel foods to help improve the transparency of this regulatory process. We held training sessions and a pilot workshop with academia, government and industry to further a tiered, risk-based approach.
As promised in the RPP, we began to develop a new regulatory approach for radiopharmaceuticals used for diagnosis and radiation therapy. We are moving forward on two regulatory initiatives. The first will address basic clinical research involving radiopharmaceuticals or positron-emitting radiopharmaceuticals. The second will require drug identification numbers (DINs) on radiopharmaceuticals.
The Department also started work, in collaboration with Agriculture and Agri-Food Canada, on a new regulatory framework for health claims for foods, including the use of logos and symbols. This will modernize the current system and support informed consumer choice with appropriately substantiated claims.
The Community of Federal Regulators is a partnership of all federal departments and agencies with regulatory roles. In order to learn from each other and accelerate innovations consistent with the Cabinet Directive on Streamlining Regulation, 12 departments and agencies signed a Memorandum of Understanding, approved a business plan, developed a learning strategy and exchanged information and best practices.
Information, Education and Outreach on Health Products, Food and Nutrition
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
13.1
|
13.9
|
13.1
|
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved adoption in making safe and healthy choices for health products, food and nutrition | Percentage of target populations using information in their decision making |
Health Canada continued to provide useful information about risks and benefits related to health products and food. For example, we conducted an educational campaign to increase consumer awareness of food safety related to meat and poultry. We completed a consultation on labeling information to reduce risks from unpasteurized juice and cider. Given concerns about health issues related to sprouted seeds and beans, we developed a policy and educational material including a page on our website 15 . A review of the risks of mercury to human health was conducted and standards and consumption advice updated on limiting exposure to mercury from certain predatory fish. We also completed a survey of benzene levels in non-alcoholic beverages and followed up with industry to ensure that levels found in beverages aimed at children were reduced through product reformulation. We released over 100 health advisories for health professionals and consumers and also developed a strategy for consumer information.
The Department provided ongoing objective information on emerging technologies and their applications. This included a list of potential nanotechnology-based products and/or delivery systems that fall under our authority. We also developed and implemented a pilot project - a high school Biotechnology Teachers' Kit to introduce students to biotechnology, to stimulate discussion on benefits and risks associated with biotechnology products and to learn how these products are regulated by Health Canada.
In February 2007, we launched the revised Canada's Food Guide after extensive consultations with stakeholders and the public. We disseminated 6.1 million copies and 300,000 resource guides for educators and communicators. Other resources include a web component with interactive material. From the launch of the Guide until the end of March, we recorded 6,768,674 visits to the Guide website and demand for copies has exceeded supply.
We began work with the Public Health Agency of Canada (PHAC) and the World Health Organization (WHO) on an international framework to promote and support healthy eating and physical activity through schools. This framework is to be one of the tools provided by WHO to implement the global strategy on diet, physical activity and health. In partnership with the U.S. Institute of Medicine, Health Canada contributed to developing and disseminating Dietary Reference Intakes: The Essential Guide to Nutrient Requirements, a summary report on Dietary Reference Intakes (DRIs), available in both official languages. The DRIs underpin all nutrition programs and policies in Canada and are used in risk assessment, standard-setting and policy development. A web-based Interactive Nutrition Label and Quiz helps Canadians make informed food choices and draws continued attention to nutrition labeling.
Health Canada continued to distribute the Canadian Adverse Reaction Newsletter and publish it in the Canadian Medical Association Journal. There was also a 19 percent increase in MedEffect Canada e-Notice subscribers.
15 http://www.hc-sc.gc.ca/iyh-vsv/food-aliment/sprouts-germes_e.html
http://www.hc-sc.gc.ca/fn-an/securit/facts-faits/rawmilk-laitcru_e.html
http://www.hc-sc.gc.ca/iyh-vsv/food-aliment/juice-jus_e.html
http://www.hc-sc.gc.ca/fn-an/label-etiquet/meat-viande/index_e.html
http://www.hc-sc.gc.ca/fn-an/label-etiquet/allergen/index_e.html
http://www.hc-sc.gc.ca/fn-an/label-etiquet/nutrition/index_e.html
Monitoring safety and therapeutic effectiveness and risk management
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
104.9
|
111.2
|
104.9
|
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Strengthened vigilance over safety and therapeutic effectiveness for health products and food on the market | Overall rating of Health Canada's post-market safety and therapeutic effectiveness activities | We are working on developing a new indicator |
We followed through on our RPP commitments under this sub-activity. For example, we carried out nutrition-related surveillance activities such as posting a guide to accessing and interpreting Canadian Community Health Survey data on our website, as well as printing and distributing the guide to stakeholders.
The Department received $190 million and $170 million over five years through the 2003 and 2005 budgets, for the Therapeutics Access Strategy and the Therapeutic Product Safety Initiative, respectively. These funds have enabled us to enhance post-market surveillance of drugs and other therapeutic products. For example, the Therapeutics Access Strategy has facilitated the reduction in backlog reports while the Therapeutic Product Safety Initiative has increased our scientific/medical capacity to monitor and evaluate safety information and act decisively to protect Canadians.
In 2006, we awarded a contract to develop a more sophisticated Adverse Reaction Reporting System that will collect and analyze adverse reaction information more effectively. In 2007-2008, the system will focus on post-market adverse reactions to pharmaceuticals, biologics and natural health products. It will later be expanded to include pre-market Adverse Reaction Reports from clinical trials.
We consulted on possible Environmental Assessment Regulations for substances regulated under the Food and Drugs Act to minimize the effects of therapeutic products on the environment, beginning with pharmaceuticals, veterinary drugs, radiopharmaceuticals, medical devices and cosmetics.
Health Canada's Inspectorate Quality Management team, managed within the Quebec Region, conducted four evalua-tions of international mutual recognition agreements covering the Good Manufacturing Practices of drug/medici-nal products with the Czech Republic and Hungary. The regulatory authorities in these two countries are now seen as equivalent relating to the mutual recogni-tion agreements pertaining to drugs and other medicinal products.
Health Canada conducted 51 clinical trial inspections, although our target was 60. This represents 1.2 percent of clinical trials, versus the targeted 1.5 percent. Our goal is to achieve the international level of 2 percent in future years, as advocated by the Standing Committee on Health in 2004. The challenge has been the availability of trained inspectors to perform Good Clinical Practices (GCP) inspections, especially given the time required to train new inspectors and competing priorities. Of the 51 clinical trials, 49 were rated compliant (96.1 percent), indicating that subjects in these trials are not exposed to undue risks and that trials are generally compliant with Canadian regulations.
We conducted 144 inspections of medical device establishments representing a 62 percent increase from the previous year. This fell below our target of 170 for two major reasons: more resources were required for inspection follow-up and to deal with medical device incidents that arose during the year. Nevertheless, we still made a significant improvement in the number of medical device establishments inspected, as illustrated in the figure below. 19
Medical Device Establishment Inspections Conducted by Fiscal Year
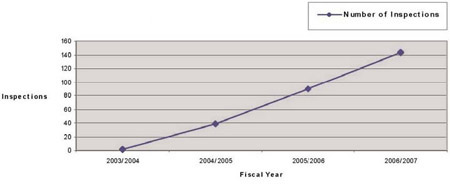
In 2006, the Health Portfolio received funding over five years to improve preparedness for avian and pandemic influenza. Health Canada has used this funding to develop a guidance document that should accelerate access to available pandemic drugs.
We received 15,295 domestic Adverse Reaction Reports (compared to 15,107 in 2004-2005 and 14,868 in 2005-2006). Also received were 10,686 domestic reports of suspected adverse reactions to pharmaceuticals, biologics, natural health products and radiopharmaceuticals. An initial report and all subsequent information received as follow-up reports are combined and considered to be one case. Most domestic cases were reported by health professionals.
19 http://www.hc-sc.gc.ca/dhp-mps/compli-conform/index_e.html
Transparency, Public Accountability and Stakeholder Relationships
Financial Resources (millions of dollars):
|
Planned Spending
|
Authorities |
Actual Spending
|
|---|---|---|
|
18.3
|
19.5
|
18.4
|
|
Expected Results
|
Performance Indicators
|
Results
|
|
Improved public confidence and trust in the safety of health products, food and the regulatory system Level of public confidence in safety of health products, food and nutrition |
Level of public confidence in safety of health products, food and nutrition Percentage of stakeholders who hold a positive view on HPFB's transparency and openness regarding regulation of health products and food |
We are working on developing a new indicator We are working on developing a new indicator |
In recent years, Canadians have sought increased access to information and decision making about the processes through which food and therapeutic products are regulated. Accordingly, we developed and launched a policy on public input in the review of our regulated products. The policy sets out when Health Canada is expected to seek public input, what information will be disclosed to allow for informed participation, and how public input will be incorporated in a decision about a product. We expect this process to improve the credibility and quality of decisions.
In summer 2006, the Department held consultations on the Blueprint for Renewal initiatives described earlier. We posted a discussion document and electronic workbook on our Blueprint website. More than 300 stakeholders provided views on the vision and objectives. We also held regional meetings as well as consultations on Blueprint themes and initiatives such as the proposed progressive licensing framework, a policy on public input in the review of regulated products, a renewed external charging framework and a regulatory modernization strategy for food and nutrition.
There is no requirement in Canada for sponsors of clinical trials to disclose publicly the existence or results of those trials. Because disclosure of clinical trial information in a publicly available registry would increase access to that information, we convened the first meeting of an External Working Group to develop preliminary options for registration in Canada. This built on international and domestic initiatives as well as results of public consultations that we held in 2005. We developed an online workbook to get public input on policy options, later posting the results on our website. Also posted were the External Working Group's final report and recommendations on how to proceed with registration and disclosure of clinical trial information in Canada.
Strategic Outcome #3(a): Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments
Program Activity Name: Healthy Environments and Consumer Safety
In 2005 we opened our Office of Paediatric Initiatives as a focal point for an integrated approach to health and safety issues affecting children, from food and nutrition to drugs and vaccines and other therapeutic and diagnostic health products. We have invited stakeholders to identify nominees for the soon-to-be established Paediatric Expert Advisory Committee on Health Products and Food.
Expected Results:
- Reduced risks to health and safety, and improved protection against harm associated with workplace and environmental hazards and consumer products (including cosmetics)
|
Performance Indicators
|
Results
|
|
Percentage of federal public employees remaining at work through and following an injury, illness or traumatic incident |
84% of federal workers who had to leave work for more than 13 weeks returned to work. |
| Treasury Board Secretariat Statistics on leave, accommodation and injury in the workplace | Received 4,747 requests for ergonomic assessments, completed 96%; received 2,376 requests for Fitness to Work health evaluations, completed 90%. |
| Level of client satisfaction with occupational health and contingency planning services | Employee Assistance Program scored above 80% on surveys and interviews, including follow-up surveys that take place three months after service. |
| Client satisfaction surveys | 97.2% of respondents are satisfied with the Employee Assistance Program |
| Percentage of Canadians who are aware that their health can be affected by environmental factors | Development of baseline data taking place. |
|
Reported incidents of product-related deaths and injuries associated with: consumer products; cosmetics; workplace chemicals; new chemical substances; products of biotechnology; radiation-emitting devices; environmental noise; solar UV radiation Reduced health and safety risks associated with tobacco consumption and the abuse of drugs, alcohol and other substances |
Development of baseline data taking place. |
| Prevalence of drug and substance abuse in Canada. Canadian Alcohol and Drug Use Monitoring Survey | Enhanced capacity to assess prevalence of substance use and abuse is being developed. |
| Smoking prevalence in Canada.Reduction in smoking prevalence from 25% to 20% Canadian Tobacco Use Monitoring Survey | 19% of the population, aged 15 years and older smoke. |
Financial Resources (millions of dollars):
| Planned Spending |
Authorities
|
Actual Spending
|
|---|---|---|
|
289.9
|
305.3
|
294.1
|
Human Resources (FTEs):
| Planned |
Planned
|
Difference |
|---|---|---|
|
1,956
|
1,950
|
6
|
Healthy Environments and Consumer Safety activities are founded in legislation that includes the Food and Drugs Act, Controlled Drugs and Substances Restraint Exercise Act, the Hazardous Products Act, the Radiation Emitting Devices Act, the Canadian Environmental Protection Act and the Tobacco Act. These Acts encompass diverse elements such as drinking water quality, air quality, radiation exposure, products of biotechnology and of other new and emerging technologies (including nanotechnology), substance use and abuse (including alcohol), consumer product safety, tobacco and second-hand smoke, workplace health, and chemicals in the environment and the workplace. We are also engaged in the Government's public safety and antiterrorism initiatives, inspection of food and potable water for the travelling public and health contingency planning for visiting foreign dignitaries.
Explanation of the above financial information:
- Variances between planned spending versus total authorities are mainly due to:
- Contributions to the government-wide 2006-2007 $1 billion Spending Restraint Exercise
- Funding from Management Reserve - Public Service Health Program
- Funding from Management Reserve - Litigation Management.
Actual spending is $11.2 million lower than total authorities mainly due to:
- Year end adjustments of Department of Justice expenditures
- Other operating lapses in various programs.
We seek to achieve our priorities through: enhanced compliance with regulations, standards and guidelines; increased awareness of regulated health products; action related to healthy and safe living, working and recreational environments; enhanced involvement of stakeholders; and improved scientific knowledge and capacity to support decision making. We collaborated extensively with partners and stakeholders inside and outside Canada, had an active presence in every region and used risk management processes to identify priorities for action. The Department fulfilled its responsibilities in accordance with sustainable development prin-ciples in order to achieve economic, social, cultural and environmental objectives.
We had success in fulfilling our plans and priorities as set out in the RPP and in addressing new Government priorities that emerged such as the Clean Air Agenda and Environmental Agenda.
In response to concerns raised by the Office of the Auditor General, Health Canada has implemented policies and procedures to improve accountability and stewardship. We have developed short-term and longer-term strategies and priorities for reducing health and environmental risks to Canadians.
Our achievements and challenges in the five sub-activities under this program activity are described below.
Safe Environments Program
Financial Resources (millions of dollars):
|
Planned Spending
|
Authorities
|
Actual Spending
|
|---|---|---|
|
83.5
|
86.1
|
85.0
|
| Expected Results |
Performance Indicators
|
Results
|
|---|---|---|
| Availability and Canada-wide adoption of measures to control the risks to human health posed by environmental contaminants |
Percentage of completion of legislated |
Baselines being established |
| Increased knowledge, understanding and involvement by Canadians in environmental health issues |
Percentage of Canadians who are aware that their health can be affected by environmental factors | |
| Science-based decision making within Canada regarding health risks of environmental contaminants | ||
| Improved scientific knowledge and capacity within the Canadian scientific community and international collaboration on environmental health issues to ensure that Canadians have increased confidence in environmental health information and protection mechanisms |
We continued to identify and manage health risks posed by environmental factors in living, working and recreational environments. The scope of activities included: drinking water quality, climate change, clean air programming, contaminated sites, toxicology, and regulatory activities. The Department was also involved in risk assessment and management of chemical substances, microbiological pathogens, environmental noise, environmental electromagnetic frequencies, solar ultraviolet radiation, as well as preparedness for nuclear and environmental disasters. Research related to these activities and to exposure assessment, hazard identification, mechanistic and population studies supported our science-based decision making.
We were involved in developing elements of the Government's Clean Air Agenda and Environmental Agenda. Collaboration with Environment Canada led to a comprehensive strategy to address air emissions from indoor and outdoor sources. Included is a regulatory approach to emissions of greenhouse gases and air pollutants caused by fuels and consumer and commercial products and those generated by industrial sectors such as electricity generation, upstream oil and gas, pulp and paper, and chemicals.
We carried out our responsibilities under the Canadian Environmental Protection Act, including completing identification and prioritization (“categorization”) of the 23,000 substances on the Domestic Substances List (DSL). About 4,000 were identified as requiring further action. Of these, 1,200 were identified as low priority, 2,600 as medium priority, and 200 as high priority.
The Alberta Region, in collaboration with the Alberta Heritage Foundation for Medical Research, the Alberta Centre for Child, Family and Community Research and key Alberta part- ners and stakeholders, hosted a 2006 Child, Health and the Environment symposium. The subsequent work of the Alberta Child Health and Environment Advisory Group led to recommendations for leaders and champions in Alberta on needed research in this area. As well, the group offered advice to the CanadianCMP Partnership on Child Health and the Environment's third workshop - Research Informing Policy.
Based on these categorization results, Health Canada developed, in conjunction with Environment Canada, the Chemicals Management Plan (CMP), a key component of the Government's Environmental Agenda. The CMP will build on Canada's position as a global leader in safe management of chemical substances and products, with a focus on timely response to environmental and health threats. The CMP enables integration and coordination of the Canadian Environmental Protection Act (CEPA) with other federal legislative tools, such as the Food and Drugs Act, Hazardous Products Act and the Pest Control Products Act. In addition to increasing health and environmental Childresearch, monitoring and tracking, the CMP provides an opportunity for industry and other stakeholders to inform the decision-making process. The CMP sets recomtimelines between now and 2020 for addressing the 4,000 substances that met categorization criteria. The CanadianCMP places the onus on industry to provide the Government with information about how they are safely managing 200 high priority chemical substances. The Government is separating those into batches of 15 to 30 substances and is publishing a batch in the Canada Gazette every three months. The first batch was published in February 2007.
The Department worked with provincial and territorial governments on drinking water quality, including finalization of four Drinking Water Quality Guidelines. In partnership with the Public Health Agency of Canada, we began development of a drinking water advisory system to help track Boil Water Advisories and their sources. We also developed a protocol for a coordinated and systematic federal approach to dealing with outbreaks of water-borne illness and contamination of drinking water.
Health Canada continued its involvement in developing an Air Health Indicator (AHI) for 2008 that will evaluate trends in air quality and associated health impacts over time. We also rolled out the pilot version of the Air Quality Benefits Assessment Tool (AQBAT). It was used to estimate health impacts for the Climate Change and Health Vulnerability Assessment, the benefits of gasoline blends with 10 percent ethanol, and various scenarios under potential new regulations to address air pollution. The Border Air Quality Strategy (BAQS) was completed and included an Air Quality Health Index and two regional pilot studies. One looked at impacts of air pollution on the health of children and other vulnerable populations.
Departmental nuclear emergency response capabilities were used to deal with three events. Canada's networks provided the only international confirmation of the underground nuclear bomb test by North Korea. We conducted analysis of radioactively contaminated mushrooms destined for the U.S. and imported via Canada from Eastern Europe. Our laboratories were involved in assessment and monitoring of over 40 Canadians deemed to be at risk of contamination from a Polonium-210 incident in London, England. Our Department also continued support for development of the Accident Reporting and Guidance Operational System (ARGOS) and the Federal Nuclear Emergency Plan electronic mapping systems as well as work to achieve more rapid processing of emergency samples. We also undertook preliminary analysis and evaluation of the effect of radon in Canadian households to guide the expected Radon Reduction Strategy.
Departmental contributions ensured proper scope of the human health component of the Canadian International Polar Year (IPY) program (2007-2009). Outcomes will include a better understanding of the current health disparities in the North, of the impact of climate change on health, and of community resilience to change. The Department participated in the development of new methods to assess impacts of environmental change on Arctic populations and ensured that high priority health issues will be addressed during IPY
Product Safety Program
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
31.3
|
33.1
|
31.1
|
| Expected Results |
Performance Indicators
|
Results
|
|---|---|---|
| Reduced risk of death and injury from exposure to hazardous products and substances associated with: consumer products; cosmetics; workplace chemicals; new chemical substances; products of biotechnology; radiation emitting devices; environmental noise; solar UV radiation |
Reported incidents of product-related deaths and injuries Level of exposure to hazardous products and substances associated with: consumer products; cosmetics; workplace chemicals; new chemical substances; products of biotechnology; radiation emitting devices; environmental noise; solar UV radiation Working on development of baseline data through performance measurement and the Product Safety Program Capacity Assessment Project. |
Working on development of baseline data through performance measurement and the Product Safety Program Capacity Assessment Project. |
To reduce the number of unsafe consumer products on the market in Canada, we conducted ongoing regulatory, monitoring and compliance activities. We also provided information, education and guidance to the public and industry, such as hazard and technical information to importers and manufacturers to encourage safer product design. In addition we identified unsafe products and product-related risks through research, laboratory testing and investigation, surveillance and monitoring activities and carried out regulatory and policy development. More than 12,000 inspections comprising market surveillance in 10 consumer product areas and over 4,000 customs referrals led to removal of approximately 1,600 product lines from the market. Approximately 27 contracts involving over 2,400 consumer products were tested as part of pre-market assessment to reduce the likelihood of hazardous products available on the market.
For example, we worked on child safety issues including strangulation by the cords of window coverings such as curtains and blinds. We carried out a multimedia information campaign and drafted regulations mandating performance requirements. No incidents related to this issue were reported during the year. We continued development of regulations for children's products, such as crayons, crib toys, clothing, and strollers, under the Lead Risk Reduction Strategy (LRRS). As well, final publication of revised Glazed Ceramics and Glassware Regulations achieved harmonization between American and Canadian standards.
To minimize future threats of skin cancer among today's children, the Department continued with the Sun Awareness Project, a school-based program that educates children about sun exposure. More than 100,000 students have been taught sun safety in elementary and secondary school classrooms since 2005.
Health Canada collaborated with partners in Canada and internationally to implement the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), which, in the Canadian context, deals with consumer chemical products and workplace chemicals.
The Department continued to assess the potential health risks of new chemicals such as fabric dyes and fuel additives, as well as biotechnology products. Approximately 500 new chemical notifications were received.
We completed about 850 assessment reports, significantly reducing our backlog. Risk management actions were renewed for four new perfluorinated chemical substances (commonly used as water and grease repellants for materials such as paper, textiles, carpet and leather, or in the manufacturing process for non-stick coatings on items such as pots and pans), and additional information-gathering conditions were imposed on 12 other new substances.
Weblinks: Product Safety Program
 Product Safety Program
Product Safety Program- Lead Risk Reduction Strategy
- CEPA (Canadian Environmental Protection Act)
- Consumer Product Safety
Workplace Health and Public Safety
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
29.9
|
40.5
|
40.5
|
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Reduced risks to health and safety and improved protection against harm associated with workplace and environmental hazards and consumer products |
Level of client satisfaction with occupational health and contingency
|
|
| Level of reported incidents of deaths and injuries associated with: workplace chemicals; new chemical substances; radiation emitting devices; and environmental noise |
|
|
| Statistics on leave, accommodation, and injury in the workplace |
|
|
| Healthy Public Service |
Percentage of federal public |
|
| Client satisfaction with occupational health and contingency planning services |
|
|
| Improved public health for the travelling public | Percentage occurrence of incidents of gastrointestinal diseases on cruiseships with a target of less than 2% of passengers and crew |
|
The Workplace Health and Safety Program (WHPSP) provides occupational health and safety services to approximately 200,000 federal government employees, protects the health and safety of dignitaries visiting Canada, and promotes health and safety in workplaces. WHPSP also inspects and assesses cargo and passenger conveyances to protect travelling Canadians.
The Department contributed to the health and safety of federal workers by responding to:
- requests for occupational health and safety services;
- ergonomic service requests;
- Employee Assistance Program (EAP) consultations; and
- workplace investigations and consultations.
Survey respondents expressed a high level of satisfaction with the Employee Assistance Program.
To improve the health of travelling Canadians, Health Canada is collaborating with the Public Health Agency of Canada (PHAC) to prepare for and manage events that may require quarantines. Educational material on virus prevention in tourism is a joint effort between the Department and municipal health authorities. We are additionally responsible for International Health Regulations under Canada's obligations to the World Health Organization (WHO). Health Canada continued to inspect ships, aircraft and passenger trains and ancillary services, achieving the results in the table above. In collaboration with the U.S. Centers for Disease Control and the cruise industry, we are combatting the spread of norovirus among travelers through inspections and the reporting of illness in advance of arriving at Canadian ports.
The Department continued to fulfill its obligations for protecting the health and well-being of foreign dignitaries and federal public servants. We organized 114 health plans for visiting dignitaries, including the Prime Ministers of Australia, Japan and senior U.S. government officials. Work on future requirements, including the Francophonie Summit and the Olympic Games in 2010, has already been initiated.
Emergency preparedness activities involving federal, provincial and municipal authorities and agencies will improve preparedness in the event of terrorist acts of a chemical, biological, radiological or nuclear nature. We received additional funding to cover our operational deficit for these activities, stabilize the remaining fiscal year, train staff and upgrade critical technical equipment.
Workplace Health and Public Safety Program
Tobacco Control Program
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
65.7
|
66.4
|
60.8
|
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Reduce smoking prevalence among the Canadian population to 20% |
Smoking prevalence rates
|
19% of the population, aged 15 years and older, smoke. |
| Reduce number of cigarettes sold in Canada by 30% |
Consumption rates - number of cigarettes sold in Canada
|
Cigarette sales declined from nearly 42 billion in 2001 to 30 billion in 2006, a decrease of close to 29%. |
Health Canada continued to lead the Federal Tobacco Control Strategy (FTCS), and worked to reduce health and safety risks associated with tobacco consumption. We developed and administered programs, and partnered with provinces, territories and stakeholder groups to reach individuals with appropriate tools, information and resources. We developed, implemented and enforced regulations pursuant to the Tobacco Act. Our research, monitoring, surveillance and reporting activities added to our knowledge base.
Manitoba and Saskatchewan Region staff in Healthy Environments and Consumer Safety pro-gram and the First Nations and Inuit Health programs collaborated with First Nations organiza-tions to adapt the Tobacco Control Program Retailer Tool kit to ensure it would have cultural significance. First Nations facilitators were trained and several Retailer Tool kit sessions were delivered to urban reserve retailers in Saskatchewan. As well, an Aboriginal tobacco project officer was employed in the region to facilitate regulatory education prior to the gradual intro-duction of enforcement activity in urban reserves. This encouraged voluntary compliance by urban First Nations retailers.
The 10-year objectives of the FTCS, which began in 2001, are to:
- reduce smoking prevalence to 20 percent, from 25 percent in 1999;
- reduce the number of cigarettes sold by 30 percent (from 45 billion to 32 billion);
- increase retailer compliance regarding youth access to sales from 69 percent to 80 percent;
- reduce the number of people exposed to environmental tobacco smoke in enclosed public spaces; and
- explore how to mandate changes to tobacco products to reduce hazards to health.
In addition to the results in the chart above, Canadian Tobacco Use Monitoring Survey (CTUMS) data collected between February and June 2006 identified a continued downward trend in smoking among Canadians aged 15 years and older. Maintaining or improving smoking levels in the over-15 population will require sustained attention. Adults aged 20-24 are an important sub-population, since young adult males report the highest prevalence for smoking (29 percent), with 20 percent smoking daily. The 2006 survey also found the rate of retailers refusing to sell tobacco products to youth was 81.7 percent. By comparison, the rate was 47.9 percent in 1995, when this was first measured.
An evaluation of the first five years of the FTCS found that the Strategy is: making progress in meeting the five objectives: cost-effective, provides good value for money; very relevant to Canadians and stakeholders alike; and has demonstrated success in several program interventions. That evaluation predicted that the first five years of the FTCS should result in health benefits and net economic benefits, such as:
- 25,500 fewer cancer, circulatory and chronic obstructive pulmonary disease cases;
- 6,300 fewer disease-related deaths;
- 153,000 fewer disabilities-adjusted life-years lost;
- $933 million increase in tobacco taxation revenue; and
- $1.46 billion decrease in direct health costs.
The FTCS evaluation found that regulatory interventions and taxation (affecting price) were the most effective policies. It had difficulty in quantifying the impacts of grants and contributions and national mass media campaigns on changing attitudes around tobacco or smoking behaviour.
We used these evaluation results to inform our directions for the subsequent four years of the Strategy. Those directions include a Framework for Action on Tobacco Control for Youth and Young Adults in conjunction with provincial and territorial partners. We also carried out the necessary research to support preparation of regulations that would ban terms such as “light” and “mild” in relation to tobacco products.
Drug Strategy and Controlled Substances
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
79.5
|
79.2
|
76.7
|
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Reduced health and safety risks associated with the abuse of drugs, alcohol and other controlled substances by managing the Controlled Drugs and Substances Act and its Regulations, and providing national leadership for Canada's Drug Strategy |
|
|
Health Canada reduces the harmful consequences associated with substance use/abuse through prevention, enforcement and treatment activities. By providing leadership for Canada's Drug Strategy (CDS), which is transitioning into the National Anti-Drug Strategy, we develop prevention strategies as well as monitor and report current and emerging drug trends to Canadians. We administer the Controlled Drugs and Substances Act, develop new or amended regulations as required, and provide analysis, scientific advice and identification services of seized controlled substances to law enforcement agencies.
According to the most recent Canadian Addictions Survey (CAS) in 2004, alcohol consumption and illegal use of drugs pose serious health threats and have an economic impact estimated at $39.8 billion or $1,267 per Canadian. Alcohol accounted for about $14.6 billion or 36.6 percent of the costs and illegal drugs accounted for approximately $8.2 billion or 20.7 percent. Risky drinking practices and the use of marijuana, cocaine/crack, LSD, Speed and heroin increased between 1994 and 2004. Prescription drug abuse involving opioids, sedatives/hypnotics and stimulants are another concern. Health Canada began to update CAS as an early step in establishing a new Canadian Alcohol and Drug Use Monitoring Survey (CADMUS). This involved work on the core questionnaire and testing of new elements concerning abuse of psychoactive pharmaceutical products.
The Interim Year 2 evaluation report on CDS was tabled and we began to address its recommendations to: streamline indicators, enhance capacity to evaluate the Drug Strategy Community Initiatives Fund (DSCIF), described below, and other programs and develop collaborative models.
The Alcohol and Drug Treatment and Rehabilitation (ADTR) Program continued to fund improved treatment for women and youth. A review of the ADTR concluded that systemic change was needed to move substance abuse treatment systems toward more evidence-informed practices. In response, we met with provincial health representatives to review the program and discuss its reorientation. Three drug treatment courts were fully implemented in Ottawa, Winnipeg, and Regina for a total of six such courts co-funded with Justice Canada. These specialized courts offer treatment as an alternative to incarceration for non-violent offenders who are addicted to cocaine or opiates.
Our drug analysis laboratories worked with law enforcement agencies to identify and analyze approximately 105,000 samples, an increase of six percent over the previous year. Our expert advice and aid led to the dismantling of 37 clandestine labs and destruction of 118,006 seized substances.
Health Canada funded 16 new projects under DSCIF, for a total of 175 projects since its inception. The DSCIF was established under the CDS for initiatives at the national, regional, provincial/territorial and local levels to facilitate community based-solutions to substance abuse problems and promote public awareness. Our $2,912,599 in contribution funding generated almost $2 million in funding from other sources. The DSCIF will be transitioning to reflect new priorities under the National Anti-Drug Strategy, through performance measurement and evaluation work already initiated.
Weblinks: Drugs and Controlled Substances

- Canada's Drug Strategy
- Medical Use of Marihuana
- Controlled Substances and Precursor Chemicals
- Health Canada's Marihuana Supply
- Drug Analysis Service
- National Framework for Action to Reduce the Harms Associated with Alcohol and Other Drugs and Substances in Canada
- Treatment and Rehabilitation
- Drug Strategy Community Initiatives Fund
- Be Drug Wise
- Canada's Drug Strategy Publications
- Overview of Alcohol and Other Drug Use in Canada
Strategic Outcome #3(b): Reduced Health and Environmental Risks from Products and Substances, and Safer Living and Working Environments
Program Activity Name: Pest Control Product Regulation
Expected Results:
- Protected health and environment
- Increased use of reduced-risk pest management practices and products
- Increased public and stakeholder confidence in pesticide regulation
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Access to safer pesticides | Number of new reduced-risk active ingredients available for use in Canada | 5 |
| Percentage of reduced-risk chemicals and percentage of biopesticide active ingredients registered/pending registration in the U.S. that are registered/pending registration in Canada | 71.7% reduced-risk chemicals and 32.5% biopesticides | |
| Number of new active ingredients registered through the PMRA/U.S. EPA Joint Review or work share program | 4 | |
| Number of active ingredients addressed through reevaluation | 10 (244 cumulatively) | |
| Strengthened compliance with the Pest Control Products Act (PCPA) and Regulations | Feedback from public and stakeholders | positive |
| Users informed of reduced risk practices | Number of proposed and final regulatory decisions posted on the website | 38 |
| Transparency of pesticide regulation | Implementation of reading rooms and adverse effects reporting | Implementation complete, no adverse effects requests/reports recieved |
| Improved regulatory efficiencies and cost effectiveness | Efficiency gains achieved through electronic processes and harmonization permit the integration of new science policies and methodologies | Reduction of paper volume, centralized information repository supports more efficient delivery of transparency requirements, automation of Confidential Business Information (CBI) and privacy identification and segregation. |
| Informed public and stakeholders | Feedback from public and stakeholders | positive |
| Number of web hits | 749, 566 | |
| Number of responses provided to the public through the Pest Management Information Service | 6,000 |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
51.6
|
68.0
|
62.7
|
Human Resources (FTEs):
| Planned | Actual | Difference |
|---|---|---|
|
652
|
574
|
78
|
A significant achievement was the coming into force of the Pest Control
Products Act (PCPA) which strengthened the legal framework for pesticide regulation. Highlights include additional authorities to protect human health and the environment, increased transparency and greater post-market controls.
Explanation of the above financial information:
Variances between planned spending versus total authorities are mainly due to:
- Funding from Management Reserve - Pesticides Regulatory
- Funding for Collective Agreement
The actual spending is $5.3 million lower than total authorities mainly due to:
- Additional collection of revenue well above the historical trend
- Year end adjustments of Department of Justice expenditures.
Many of the targets set in the RPP centred on supporting implementation of the new PCPA, in particular to ensure that stakeholders were well-informed about the features and requirements. Where possible, we incorporated stakeholder feedback to ensure our services would meet their needs. Consultation with our Advisory Council also guided our actions.
International regulatory cooperation was another priority. We worked with member countries of the Organization for Economic Cooperation and Development (OECD), including the United States and Mexico, to increase availability of reduced-risk pesticides for Canadian growers without compromising human health and the environment. We also consulted with and shared scientific knowledge and best practices with other departments, provincial and territorial governments and regulators in other countries.
The Department continued to recruit and retain employees through the Pest Management Regulatory Agency Science Development Program. We reviewed this program, which is aimed at biologists and chemists, and began improvements so that we will have the specialized staff we need to deliver results.
The pesticide regulation program activity encompasses five sub-activities. Achievements under each of the sub-activities are outlined below.
Regulatory Improvement
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
6.0
|
8.3
|
7.8
|
The PCPA which came into force in June 2006 increased the transparency of our activities, enabled greater public participation, expedited registration of lower risk products and developed a new administrative Maximum Residue Limit setting process designed to protect human health and the environment.
We have streamlined the evaluation needed to support registration of lower risk products. To increase the efficiency of our process, we conducted 38 consultations with companies before they sought formal approval of new registrations for lower risk products. We also collaborated with counterparts internationally to develop a regulatory approach for these lower risk pesticides. As well, we finalized a new formulants policy that encourages the use of less toxic ingredients. In our work on the Government's Chemicals Management Plan, we participate in risk assessment and risk management of high priority chemical substances used in pesticide products.
To support transparency requirements of the PCPA, our new electronic Public Registry gave the public access to information about new applications to register or amend pest control products, evaluation reports and conditions of registrations of newly registered or reevaluated pesticides. The PCPA incorporates into law our practice of providing the public the opportunity to comment on proposed major registration decisions.
Mandatory Sales Information Reporting Regulations as well as mandatory Incident Reporting Regulations were published. These provide information to the public about registered pesticides in the Canadian marketplace.
Enhancements to our online services included a Pesticide Products Label database that is updated in real time, and a new Incident Reporting function. Effective electronic submission tools have resulted in over 80 percent of applications being submitted electronically.
New Pest Control Product Registration and Decision Making
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
25.6
|
33.6
|
27.5
|
Before new pesticide products can be registered for use in Canada, Health Canada conducts extensive pre-market assessment to ensure their use poses no unacceptable risks. Using modern scientific methods and international best practices, we conducted human health, safety and environmental risk assessments, as well as value assessments.
We continued to work closely with our counterparts internationally on a joint review program. We commenced two joint reviews with other OECD member countries. The NAFTA joint review program has resulted in 21 joint reviews and seven work shares, facilitating access to 11 conventional and newer reduced-risk chemicals. The first NAFTA pesticide label was approved simultaneously by Canada and the U.S., allowing for free movement of that pesticide between the two countries. The expansion of products with NAFTA labels strengthens competitiveness of North American growers without compromising Canada's high standards for human health and the environment.
Registered Pest Control Products Evaluation and Decision Making
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
9.8
|
12.8
|
12.1
|
Health Canada has a responsibility to reevaluate older pesticides on the market to determine if their use is acceptable in consideration of modern information requirements and scientific assessment methods. We made 17 final reevaluation decisions, 12 proposed decisions and four interim decisions. This brought our cumulative total to 244 pesticides addressed since 2001, or 61 percent of the pesticides in the reevaluation program. At the request of stakeholders, we published a reevaluation status table for the 401 pesticide active ingredients, providing them with regular updates.
Compliance
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
7.6
|
10.0
|
11.2
|
Health Canada has the responsibility to promote, maintain and enforce compliance with the PCPA. We conducted 437 investigations and delivered 13 compliance programs, assessing levels of compliance in blueberry, grape and head lettuce growers; lawn care applicators; and importers of an own-use product. We worked with provincial and other federal regulators and built on our efforts to develop performance indicators for compliance.
Pesticide Risk Reduction in Agriculture
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
2.6
|
3.3
|
4.1
|
In partnership with Agriculture and Agri-Food Canada (AAFC), we developed and implemented commodity-based risk reduction approaches that included two new crops (blueberry and raspberry). Health Canada and AAFC also coordinated pre-submission consultation meetings with U.S. pesticide registrants who own microbial and low risk alternatives. We worked with several industrial sectors to integrate sustainable pest control into their respective strategies for issues such as the Mountain Pine Beetle in the forestry sector and the Richardson's ground squirrel population in the Prairies. As well, we promoted sustainable pest management to municipal associations and at homeowner trade shows.
 Weblinks:
Weblinks:
Strategic Outcome #4: Better Health Outcomes and Reduction of Health Inequalities between First Nations and Inuit and Other Canadians
Program Activity Name: First Nations and Inuit Health
Expected Results:
The objective of the First Nations and Inuit health program activity is to improve health outcomes, by ensuring the availability of, and access to, quality health services, and supporting greater control of the health system by First Nations and Inuit.
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
2,119.1
|
2,126.0
|
2,088.0
|
Human Resources (FTEs):
| Planned | Actual | Difference |
|---|---|---|
|
2,884
|
2,993
|
109
|
Explanation of the above financial information:
Variances between planned spending versus authorities are mainly due to:
- Funding for Federal Contaminated Sites Action Plan
- Contributions to the Government-Wide 2006-2007 $1 Billion Spending Restraint Exercise
- Funding for Non-Insured Health Benefits Program
- Funding for Indian Residential Schools Program.
The actual spending is $38 million lower than authorities mainly due to:
- Delays in Indian Residential Schools Program
- First Ministers and Aboriginal Leader Meeting reprofiles
- Contributions to the Government-Wide 2006-2007 $1 Billion Spending Restraint Exercise
- Operating Budget carryforward in NIHB
- Transfers for capital projects to PACR.
Since development of the RPP, Health Canada has continued to work with First Nations and Inuit and other health partners in delivering programs
and services under the four key priorities.
Continued health-related programs and services
In partnership with First Nations and Inuit, we provided primary health care services in approximately 200 remote communities, and home and community care services in about 600 communities. We directly employed 670 nurses in First Nations communities. Including nurses funded by Health Canada but employed by First Nations, the nursing work force is approximately 1,100. These nurses deliver health services through nursing stations, community health centres, and other health facilities.
Through our Regional Offices and in partnership with First Nations and Inuit, we delivered community programs that focused on children and youth, mental health and addictions, environmental health, and commu-nicable and chronic disease prevention and management. These supplemented health services that provincial, territorial and regional health authorities provided. Through the Non-Insured Health Benefits (NIHB) Program, we provided additional benefits to eligible First Nations and Inuit regardless of their place of residence. These include coverage for drugs, dental care, vision care, medical supplies and equipment, short-term crisis intervention mental health services, and medical transportation.
The Department faces the same challenges as other health care providers such as increasing costs, demand for new health technologies, human resource shortages and an aging population. The First Nations and Inuit health system has additional challenges due to rapidly growing populations, higher than average rates of disease and injuries, and populations living in small communities, often in rural and remote areas.
Improving First Nations and Inuit health outcomes also requires action on broader determinants of health, such as economic development, education, housing and culture, so that communities can become sustainable, culturally strong and economically viable.
| Expected Results | Performance Indicators | Results |
|---|---|---|
|
Strengthened community programs |
Life expectancy (at birth, on and off reserve) |
While still behind the Canadian average, life expectancy for First Nations has increased. In 1980, First Nations males’ life expectancy was 60.9 years, and females’ was68.0 years. By 2001, life expectancy was estimated at 70 years for males and 76 for females. |
| Better health protection | Infant Mortality Rates | Infant mortality rates for First Nations have exhibited a downward trend. The Department is working with stakeholders on improved methodology for tracking this data. |
| Improved primary health care | Birth weight | Statistics for 2000 indicate that 4.7% of First Nations births are classified as low birth weight compared with 5.6% in Canada overall. The high birth weight rate for the First Nations is 21%, almost double the Canadian rate of approximately 13%. Health Canada continues to work in maternal and prenatal health to improve outcomes. |
| Access to Non-Insured Health Benefits.(NIHB) |
NIHB client utilization rates |
NIHB utilization rates represent those clients who receive at least one pharmacy benefit paid through the Health Information and Claims Processing Services system, as a proportion of the total number of clients in the fiscal year. In 2005-2006, the national utilization rate for the pharmacy benefit was 65%. Regional rates ranged from 76% in Saskatchewan to 47% in the N.W.T. and Nunavut. |
Improving quality of and access to health-related programs and services
Health Canada worked towards seamless integration of services; increasing the number of Aboriginal health professionals; supporting First Nations accreditation; improving community dental care capacity; and capital improvements and investments.
Through the community health component of the Labrador Innu Healing Strategy, Atlantic Region staff collaborated with the
Mushuau Innu First Nation in completing a functional and capital plan for a healing lodge
and wellness facilities, planned to
open by June 2007.
Labrador-based staff collaborated with the
Sheshatshiu Innu First Nation to survey approximately 400 community members. The information gathered will be used to plan health prevention and intervention delivery in line with the community's needs. The two groups also collaborated on a training plan for addictions treatment staff to enhance skills and reduce turnover.
We implemented the Aboriginal Health Transition Fund (AHTF). The Fund is designed to improve access to and quality of health services for Aboriginal peoples through better adaptation of provincial and territorial health services and their integration with federal health programs. Examples of achievements include agreements for integration projects in the provinces and territories, and communication activities to build First Nations' awareness of health services. AHTF projects support joint community health planning; coordination and co-location of health services; improved health information management, including better immunization databases and patient charting; increased access to clinical knowledge; and collaborative work on suicide awareness and response. Two advisory groups have been created, with representation from all five National Aboriginal Organizations, provinces and territories, and the federal Health Portfolio.
The Health Integration Initiative, which ended in March 2006, funded eight pilot projects. The national evaluation report, which is expected to be submitted for approval in fall 2007, found that this Initiative led to sustainable partnerships. Projects under AHTF will build on the achievements and lessons learned.
We initiated work to increase Aboriginal students' awareness of health careers; partnered with other stakeholders to improve retention of health care workers in Aboriginal communities; and increased support programs available to Aboriginal health care students. Achievements include: dissemination of career awareness materials to isolated communities using video conferencing and telehealth technologies; facilitating changes to curricula of schools of medicine and nursing; and establishment of First Nations and Inuit capacity for health human resource planning in all First Nations and Inuit provincial, territorial and land claim organizations. Funding totalling $2 million was provided to the National Aboriginal Achievement Foundation to provide bursaries and scholarships for Aboriginal health career students, and to promote health careers in First Nations and Inuit schools. An agreement negotiated with the Métis National Council will provide $10 million in bursary and scholarship funding for Métis health career students over the next four years.
Our nurses were supported with professional training and educational resource materials. Training materials include a Primary Care Clinical Practice Guidelines manual, a self-study guide, and an education program for Emergency Labour Competency. These prepare nurses to transition more readily to using an Electronic Health Record system in First Nations communities. National Nursing Security and Awareness Training sessions were also conducted and training materials developed.
The Department launched the Nursing Portal in June 2006 at the Canadian Nurses Association Biennial Conference. This bilingual electronic gateway provides nurses access to an array of resources and services to support nursing practice. Planning continued on enabling Health Canada, as an employer of nurses working in First Nations communities, to post specific employer information, provide education courses and customize clinical resources.
First Nations Community Health Services, National Native Alcohol and Drug Abuse Program Treatment Centres, and Youth Solvent Abuse Treatment Centres have been pursuing accreditation with the Canadian Council on Health Services Accreditation for a number of years. There are 49 First Nations health organizations in the process of being accredited or already accredited. This ensures that First Nations health facilities offer service comparable to accredited provincial/territorial health services.
The Children's Oral Health Initiative was implemented in 140 First Nations communities and services were provided to 8,000 children.
To improve the working environment of clients and staff, and to enhance the quality of health care services, we constructed 14 health facilities, expanded six health facilities, and completed four major recapitalization initiatives.
Given the importance of environmental management in the Departmental Sustainable Development Strategy, we spent approximately $2 million to: conduct pilot Environmental Compliance Audits and Environmental Site Assessments; assess a waste water system to determine whether a new system is needed; upgrade a clinic crawlspace at Norway House Hospital in response to mould; conduct comprehensive microbial investigations at three health facilities to determine the extent and severity of mould contamination; monitor health facilities with previous mould issues; and perform a water well seal integrity study at four wells in Alberta as pilot projects for a larger project.
Promoting healthy living and disease prevention
Health Canada continued to focus on enhancing and strengthening maternal and child health, mental wellness, suicide prevention, prevention of chronic disease, communicable disease readiness and safe drinking water.
The Department funded 40 Maternal and Child Health programs in First Nations communities and supported other communities to provide the program in subsequent years. Activities included: hiring and training family visitors; “train the trainer” postpartum home visit training for 40 nurses and development of a screening catalogue and case management tool kit. In February 2007, Health Canada launched a Healthy Pregnancy campaign to provide women with information to make healthy lifestyle choices before and during pregnancy. Two distinct Healthy Pregnancy campaigns were developed to meet the needs of First Nations and Inuit communities.
Approximately 9,000 children participated in the Aboriginal Head Start On-Reserve (AHSOR) Program. We provided training for outreach and home visit workers in smaller communities and for asset mapping, family support and nutrition. The Department also enhanced the AHSOR capital infrastructure by spending $7.6 million to support capital projects. Seventeen Early Childhood Development single-window service delivery demonstration projects in First Nations communities tested the impact of streamlined funding, program reporting and community development.
A Mental Wellness Strategic Action Plan was developed, and the stakeholder approval process is under way. In addition, 60 community-based Aboriginal Youth Suicide Prevention projects were funded and implemened.
Through the Health Integration Initiativeproject in Nunavut, Health Canada's Northern Region, the Government of Nunavut and Nunavut Tunngavik Incorporated collaborated on identifying actions to better integrate federal and territorial programs and services in maternal and child health, addictions and mental healthand oral health. This was the first tripartite health project to be managed and coordinated by an Inuit organization in Nunavut, enabling it to be delivered from an Inuit perspective.
In November 2006, the Department received approval to provide professional counselling, emotional and and cultural supports to eligible former students of Indian Residential Schools under the new Indian Residential Schools Settlement Agreement.
Activities to advance a strategic and comprehensive approach to other chronic diseases in addition to diabetes were also undertaken. For example, an analysis of effective interventions to prevent chronic disease in indigenous populations was finalized and community-based models for integrated chronic disease prevention and management were developed and supported.
Pandemic influenza plans were drawn up to support First Nations communities in local preparedness and ensure better regional communicable disease emergency coordination with provinces, territories and stakeholders. A stockpile of personal protective equipment was replenished for use by front-line workers in First Nations communities in communicable disease emergencies. Health Canada provided support to the Assembly of First Nations to pilot and test a culturally-appropriate approach to pandemic planning in three First Nations communities.
By March 2007, over 40 percent of drinking water distribution systems, cisterns and community wells were routinely monitored for bacteriological contaminants as recommended in the Guidelines for Canadian Drinking Water Quality. A total of 153,604 water samples were analyzed in First Nations communities, an increase of 29 percent from last year. Health Canada is working with First Nations leadership to help communities improve their understanding of and response to Drinking Water Advisories. Six out of our seven regions now have water databases in place to monitor sample results. No instances of water-borne disease outbreaks were identified.
The British Columbia Region continues working toward accurate and timely reporting from all communities who are engaged in the Drinking Water Safety Program, while developing other communities. There are 136 community water technicians trained and supported to provide sampling programs for community water systems, in order to meet Canadian drinking water guidelines. Health Canada and the water technicians are also preparing for spring flood conditions.
Health Canada developed a school kit to raise awareness and instill an appreciation for the importance of clean, safe and reliable water
among First Nations children. We also developed communication products to focus on preventive activities related to Drinking Water Advisories.
Improving accountability and performance measurement
The Department undertook initiatives to support and enhance health surveillance, information analysis, research, and data collection and analysis. An Infant Mortality Working Group was created, with participation from various levels of government, National Aboriginal Organizations and academics, to address deficits in infant mortality data for First Nations, Inuit and Métis. The Group is developing several pilot projects, to be funded by Health Canada and the Public Health Agency of Canada, that will improve data quality and coverage as well as local data capacity.An integrated Aboriginal health surveillance strategy has been initiated to advance the Integrated Public Health Surveillance and Information plan. Pilot projects for enhanced health information management have been developed in the Alberta and Atlantic regions. These will improve surveillance systems in those regions while providing direction for other regional development in the long term.
A key challenge in reporting health information for First Nations and Inuit is to identify Aboriginal people from within administrative databases. Health Canada has funded and participated in an innovative Statistics Canada project to develop life expectancy estimates in Inuit-inhabited regions. This has led to new data on northern life expectancy. Health Canada is also supporting regional data infrastructure through the Health Data Technical Working Group, with representation from the National Aboriginal Organizations. This Group provides technical expertise on data and epidemiological matters and produces in-house statistics for the Department. Their major product is the Statistical Profile on the Health of First Nations in Canada.
The First Nations Regional Longitudinal Health Survey (RHS) is a “survey of First Nations developed by First Nations,” and is funded by Health Canada through contribution agreements. Results of the 2002-2003 RHS, released just prior to last fiscal year, have been shared with federal and provincial governments and First Nations to support evidence-based planning and evaluation. Discussions are ongoing on the next cycle of the RHS.
Health Canada finalized a Performance Measurement Strategy Reporting Template to support First Nations recipients in data collection at the community level beginning in 2008-2009.
In the RPP, the Department identified programs and services that were the organizational base for our initiatives across all four priorities just described. The charts below set out details regarding our use of resources, expected results, performance indicators and results achieved for each program area.
First Nations and Inuit Community Health Programs
Child and Youth programs
Maternal, infant and child health; increasing children's knowledge of language and culture; and their readiness for school are the main priorities of child and youth programs. The programs are: Aboriginal Head Start On-Reserve; Canada Prenatal Nutrition; Fetal Alcohol Spectrum Disorder; and Maternal and Child Health.
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved continuum of programs and supports in First Nations and Inuit communities | Number and percentage of communities with programs | 40 Maternal and Child Health programs have been established. Other communities have been supported to provide the program in subsequent years. |
| Increased participation of Aboriginal individuals, families, and communities in programs and supports | Number and type of participants in programs by program type | Approximately 9000 children participated in Aboriginal Head Start On Reserve programs. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
| 108.3 | 110.4 | 97.9 |
Mental Health and Addictions Programs
These programs provide culturally appropriate counselling, addiction prevention and mental wellness services that are largely delivered by Aboriginal people. They include: Building Healthy Communities; Brighter Futures; National Native Alcohol and Drug Abuse Program - Residential Treatment; National Native Alcohol and Drug Abuse Program - Community-based; Youth Solvent Abuse Program; First Nations and Inuit Tobacco Control Strategy 21 ; National Aboriginal Youth Suicide Prevention Strategy; Labrador Innu Comprehensive Healing Strategy; and Indian Residential Schools Resolution Health Support Program (formerly known as Indian Residential Schools - Mental Health Support Program).
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved continuum of programs and services in First Nations and Inuit communities | Number and percentage of communities with programs | All First Nations communities have access to National Native Alcohol and Drug Abuse Program, Youth Solvent Abuse Program and mental health programs. 60 community-based suicide prevention projects were delivered in . |
| Increased participation of First Nations and Inuit individuals, families and communities in programs and services | Number and type of participants in programs by program type | All former students of Residential Schools and their families are now able to access health supports via the Indian Residential Schools Resolution Health Support Program. 585 clients accessed professional mental health services. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
147.7
|
191.0
|
166.4
|
21 As part of the federal expenditure review, $2.5 million in 2006-2007, $8.3 million in 2007-2008, and $10.8 million in ongoing funding for the First Nations and Inuit Tobacco Control Strategy was targeted for reduction. Funding for 2007-2008 will be directed at completing initiatives and working with partners to develop a new policy approach.
Chronic Disease and Injury Prevention Programs
Over the long term, these programs will contribute to prevention of chronic disease and injuries within First Nations and Inuit communities. They include: Aboriginal Diabetes Initiative; Nutrition and Physical Activity Promotion; and Injury Prevention.
| Expected Results | Performance Indicators |
Results |
|---|---|---|
| Improved continuum of programs and supports in First Nations and Inuit communities | Number and percentage of communities with programs | More than 600 communities have access to health promotion and diabetes prevention services. Examples include: school policies emphasizing healthy snacks, children’s camps that focus on preventing diabetes through healthy lifestyles, and walking clubs to help those at risk. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
37.0
|
24.6
|
26.4
|
First Nations and Inuit Health Protection and Public Health
Communicable Disease Control Programs
These programs protect First Nations and Inuit communities from communicable diseases through measures to manage, contain and control risks of outbreaks. They include: Tuberculosis; Immunization; HIV/AIDS; and Communicable Disease Emergencies.
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved access to communicable disease prevention and control programs for First Nations and Inuit individuals, families and communities |
Number and percentage of communities with programs |
All First Nations communities on-reserve are supported Inuit communities are supported in communicable disease prevention and control through contribution agreement funding or through National Aboriginal |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
27.6
|
26.2
|
30.6
|
Environmental Health and Research Programs
These programs create and maintain healthy and safe community environments through: investigation of potential environmental health-related outbreaks; raising awareness of water-borne, food-borne and vector-borne illnesses including health problems from poor indoor air quality, such as mould. They provide for pest control and build community human resource capacity to adapt to environmental conditions, to maintain safe environments and to deal safely with hazards. These programs include: First Nations Water Management Strategy; West Nile Virus; Contaminated Sites; Transportation of Dangerous Goods; Food Safety; Facilities Health Inspections; housing; and research.
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved environmental health risk management | Number of communities with environmental health officers Number of communities equipped with water testing/sampling kits | 478 communities have dedicated environmental health officers. 546 communities are equipped with water testing/sampling kits. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
48.9
|
46.3
|
39.0
|
First Nations and Inuit Primary Health Care
Comprehensive health care services are provided to remote First Nations and Inuit settlements to supplement primary care provided by provincial, territorial and/or regional health authorities. These include emergency and acute care. Health Canada ensures links to other health care providers and/or institutions as required by the client's condition. The continuum of care includes illness and injury prevention and health promotion such as the Home and Community Care Program and the Oral Health Strategy.
| Expected Results | Performance Indicators | Results |
|---|---|---|
| Improved access to primary health care programs and services for First Nations and Inuit individuals, families and communities |
Number and percentage of communities with programs |
605 of 645 (94%) First Nations communities have access to home care services, represents 97% of eligible population. 55 (100%) Inuit communities have access to home care services, represents 100% of eligible population. |
| Number of treatment centres by type, in the communities | 54 Alcohol and Drug Abuse and 8 Youth Solvent Abuse treatment centres are in operation. | |
| Eligible client utilization rates of NIHB - Dental Benefits | In 2005-2006, the national utilization rate for the dental benefit was 37%. Regional rates ranged from 46% in Quebec to 30% in Manitoba. |
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
247.0
|
270.5
|
289.0
|
Non-Insured Health Benefits
The NIHB program provides 765,000 registered First Nations and recognized Inuit with a limited range of medically necessary goods and services not provided through private or provincial and territorial health insurance plans. The benefits include: dental care, vision care, pharmacy benefits (prescription drugs and some over-the-counter medication), medical supplies and equipment, crisis intervention, mental health counseling, transportation to medically required health services and payment of health premiums for individuals in Alberta and British Columbia.
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
966.3
|
1,018.7
|
996.4
|
Governance and Infrastructure Support
Health Governance and Infrastructure Support aims to increase First Nations and Inuit control over health programs, establish an adequate First Nations and Inuit infrastructure and services and improve capacity to generate and use health information. These activities include: health planning; capacity building; integration and coordination of health services; stewardship and health research; knowledge and information management. In November 2006, the Government signed a Memorandum of Understanding with the Province of British Columbia and the British Columbia First Nations Leadership Council. This MOU committed the parties to building a tripartite relationship for improving the health of B.C. First Nations, and led to the signing of the Tripartite First Nations Health Plan for British Columbia in June 2007.
Financial Resources (millions of dollars):
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
|
536.4
|
438.7
|
442.3
|
Weblinks:

- Non-Insured Health Benefits 2005-06 Annual Report
- Non-Insured Health Benefits Program
- Aboriginal Head Start On-Reserve
- Fetal Alcohol Spectrum Disorder
- Aboriginal Diabetes Initiative
- Injury Prevention
- Indian Residential Schools Resolution Health Support Program
- National Native Alcohol and Drug Abuse Program
- Drinking Water Quality
- Immunization Schedule for Infants and Children
- Targeted Immunization Program
- e-Health
- Aboriginal Health Human Resources Initiative
Section III: Supplementary Information
Organizational Chart
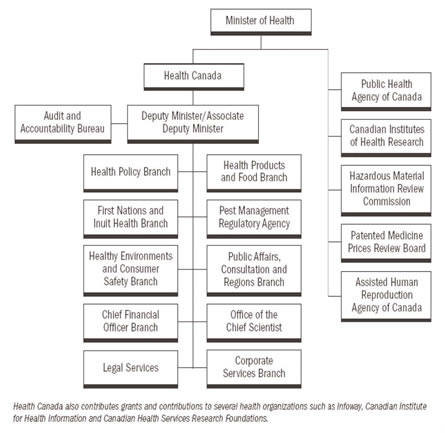
Table 1: Comparison of Planned to Actual Spending (Incl. FTEs)
This table offers a comparison of the Main Estimates, Planned Spending, Total Authorities and Actual Spending for the most recently completed fiscal year, as well as historical figures for Actual Spending.
The $35.7 million increase from Main Estimates to Planned Spending is due to anticipated funding for such initiatives as Avian or Pandemic Influenza Preparedness, Funding to the O-Pipon-Na-Piwin Cree Nation, Recognize a Landless Band and Registration of Newfoundland Indians and Non-Insured Health Benefits Program.
The $79.0 million increase from Planned Spending to Total Authorities is due new program initiatives and sustainability funding which is received through Supplementary Estimates.
The $92.6 million decrease between Total Authorities and Actual Spending is mainly the result of:
- lapse in the Health Council special purpose allotment;
- delays in Indian Residential Schools Program;
- delays in the Advertising Special Purpose Allotment;
-
- lapse of frozen allotment which includes:
- Follow-up to the Special Meeting of First Ministers and Aboriginal Leader Reprofile
- Contributions to the government-wide 2006-2007 $1 billion Spending Restraint Exercise
- Access to Medicines (Regime)
- year end adjustments of Department of Justice (DOJ) expenditures.
(millions of dollars)
| Program Activities | 2004-2005 | 2005-2006 | 2006-2007 | |||
|---|---|---|---|---|---|---|
| Actual Spending* | Actual Spending | Main Estimates | Planned Spending (1) | Actual Authorities (2) | Actual Spending (2) | |
| Health Policy, Planning and Information | 385.9 | 375.1 | 288.6 | 288.4 | 312.6 | 290.4 |
| Health Products and Foods | 261.5 | 256.9 | 262.0 | 262.1 | 278.2 | 262.3 |
| Healthy Environments and Consumer Safety | 289.9 | 277.9 | 290.7 | 289.9 | 305.3 | 294.1 |
| Pest Control Product Regulation | 59.3 | 54.6 | 51.7 | 51.6 | 68.0 | 62.7 |
| First Nations and Inuit Health | 1,820.0 | 1,927.5 | 2,082.4 | 2,119.1 | 2,126.0 | 2.088.0 |
| TOTAL | 2,816.6 | 2,892.0 | 2,975.4 | 3,011.1 | 3,090.1 | 2,997.5 |
| Less: Non-Respendable Revenue | -51.3 | -19.8 | 0.0 | -8.9 | -8.9 | -51.8 |
| Plus: Cost of services received without charge** | 58.9 | 85.6 | 0.0 | 84.7 | 84.7 | 91.9 |
| Net Cost of Department | 2,824.2 | 2,957.8 | 2,975.4 | 3,086.9 | 3,165.9 | 3,076.6 |
| Full Time Equivalents | 8,026 | 8,544 | 8,694 | 8,711 | 8,708 | 8,686 |
| 1) from the 2006-2007 Report on Plans and Priorities 2) from the 2006-2007 Public Accounts * These amounts are estimated due to the change in reporting structure from Business Line to Program Activity. However, the total number for the Department is accurate. ** Services received without charge include accommodation provided by PWGSC, the employer's share of employees' insurance premiums, Workers' Compensation coverage provided by Social Development Canada, and services received from the Department of Justice . This table excludes amounts related to the Public Health Agency of Canada (PHAC). |
||||||
Table 2: Resources by Program Activity
This table reflects how resources are used within Health Canada by appropriation and by program activity.
(millions of dollars)
| 2006 - 2007 | |||||||
|---|---|---|---|---|---|---|---|
| Program Activity | Operating | Capital | Grants | Contributions and other Transfer Payments | Total Gross Expenditures | Less Respendable Revenues | Total Net Expenditures |
| Health Policy, Planning and Information | |||||||
| (Main Estimates) | 95.6 | 57.1 | 135.9 | 288.6 | 288.6 | ||
| (Planned Spending) | 95.4 | 57.1 | 135.9 | 288.4 | 288.4 | ||
| (Total Authorities) | 125.1 | 53.2 | 134.3 | 312.6 | 312.6 | ||
| (Actual Spending) | 117.6 | 43.3 | 129.5 | 290.4 | 290.4 | ||
| Health Products and Food | |||||||
| (Main Estimates) | 291.9 | 1.4 | 5.9 | 4.0 | 303.2 | -41.2 | 262.0 |
| (Planned Spending) | 292.0 | 1.4 | 5.9 | 4.0 | 303.3 | -41.2 | 262.1 |
| (Total Authorities) | 308.5 | 1.4 | 5.7 | 3.8 | 319.4 | -41.2 | 278.2 |
| (Actual Spending) | 292.5 | 1.2 | 5.5 | 3.8 | 303.0 | -40.7 | 262.3 |
| Healthy Environments and Consumer Safety | |||||||
| (Main Estimates) | 260.4 | 1.0 | 5.1 | 39.6 | 306.1 | -15.4 | 290.7 |
| (Planned Spending) | 259.5 | 1.0 | 5.1 | 39.7 | 305.3 | -15.4 | 289.9 |
| (Total Authorities) | 275.6 | 1.0 | 5.1 | 39.0 | 320.7 | -15.4 | 305.3 |
| (Actual Spending) | 262.6 | 0.8 | 4.5 | 38.7 | 306.6 | -12.5 | 294.1 |
Resources by Program Activity continued...
(millions of dollars)
| 2006 - 2007 | |||||||
|---|---|---|---|---|---|---|---|
| Program Activity | Operating | Capital | Grants | Contributions and other Transfer Payments | Total Gross Expenditures | Less Respendable Revenues | Total Net Expenditures |
| Pest Control Product Regulation | |||||||
| (Main Estimates) | 58.7 | 58.7 | -7.0 | 51.7 | |||
| (Planned Spending) | 58.6 | 58.6 | -7.0 | 51.6 | |||
| (Total Authorities) | 75.0 | 75.0 | -7.0 | 68.0 | |||
| (Actual Spending) | 70.1 | 70.1 | -7.4 | 62.7 | |||
| First Nations and Inuit Health | |||||||
| (Main Estimates) | 1,144.7 | 1.5 | 30.0 | 911.7 | 2,087.9 | -5.5 | 2,082.4 |
| (Planned Spending) | 1,174.6 | 1.5 | 30.0 | 918.5 | 2,124.6 | -5.5 | 2,119.1 |
| (Total Authorities) | 1.192.8 | 1.5 | 30.0 | 907.2 | 2,131.5 | -5.5 | 2.162.0 |
| (Actual Spending) | 1,163.3 | 1.3 | 30.0 | 896.6 | 2,091.2 | -3.2 | 2,088.0 |
| TOTAL | |||||||
| (Main Estimates) | 1,851.3 | 3.9 | 98.1 | 1,091.2 | 3,044.5 | -69.1 | 2,975.4 |
| (Planned Spending) | 1,880.1 | 3.9 | 98.1 | 1,098.1 | 3,080.2 | -69.1 | 3,011.1 |
| (Total Authorities) | 1,977.0 | 3.9 | 94.0 | 1,084.3 | 3,159.2 | -69.1 | 3,090.1 |
| (Actual Spending) | 1,906.1 | 3.3 | 83.3 | 1,068.6 | 3,061.3 | -63.8 | 2,997.5 |
More detailed explanations on all program activities can be found in Section II: Analysis of Performance by Strategic Outcome.
Table 3:Voted and Statutory Items
(millions of dollars)
| VOTE | 2006-2007 | ||||
|---|---|---|---|---|---|
| Main Estimates | Planned Spending (1) | Total Authorities (2) | Actual Spending (2) | ||
| Health Canada | |||||
| 1 | Operating expenditures | 1,674.5 | 1,702.9 | 1,805.4 | 1,739.6 |
| 5 | Grants and contributions | 1,189.3 | 1,196.2 | 1,178.3 | 1,151.9 |
| (S) | Minister's car allowance and salary | 0.1 | 0.1 | 0.1 | 0.1 |
| (S) | Payments for insured health services & extended health care services | - | - | 0.0 | 0.0 |
| (S) | Spending of proceeds from the disposal of surplus Crown assets | - | - | 0.5 | 0.1 |
| (S) | Refunds from previous year's revenue | - | - | 0.3 | 0.3 |
| (S) | Collection agency fees | - | - | 0.0 | 0.0 |
| (S) | Court awards | 0.0 | 0.0 | ||
| (S) | Contributions to employee benefit plans | 111.5 | 111.9 | 105.5 | 105.5 |
| Total Department | 2,975.4 | 3,011.1 | 3,090.1 | 2,997.5 | |
(1) from the 2006-2007 Report on Plans and Priorities
(2) from the 2006-2007 Public Accounts
(S) indicates expenditures the Department is required to make that do not require an appropriation act.
Table 4: Services Received Without Charge
(millions of dollars)
|
ITEM
|
2006-2007
|
| Accommodation provided by Public Works and Government Services Canada |
34.9
|
| Contributions covering employer's share of employees' insurance premiums and expenditures paid by Treasury Board Secretariat |
51.0
|
| Worker's compensation coverage provided by Social Development Canada |
0.7
|
| Salary and associated expenditures of legal services provided by the Department of Justice |
5.3
|
| Total 2006-2007 Services Received without Charge |
91.9
|
Table 5: Sources of Respendable and Non-Respendable Revenue
Reflected in this table is the collection of respendable revenues by program activity/branch and of nonrespendable revenues by classification and source.
Respendable revenues refer to funds collected for user fees or for recovery of the cost of departmental services. These revenues include those both external and internal to the government, the majority being external.
A variety of respendable revenues are collected including Medical Devices, Radiation Dosimetry, Drug Submission Evaluation, Veterinary Drugs, Pest Management Regulation, Product Safety, hospital revenues resulting from payments for services provided to First Nations and Inuit Health hospitals, which are covered under provincial or territorial plans, and for the sale of drugs and health services for First Nations communities.
Non-respendable revenues are shown by source in order to reflect the information in a useful format. The Department is not allowed to respend these revenues.
(millions of dollars)
| Respendable Revenues | Actual Revenues 2004-2005 | Actual Revenues 2005-2006 | 2006-2007 | |||
|---|---|---|---|---|---|---|
| Main Estimates | Planned Revenue | Total Authorities | Actual Revenues | |||
| Program Activity/Branch | ||||||
| Health Products and Food Health Products and Food Branch | 35.1 | 37.7 | 41.2 | 41.2 | 41.2 | 40.7 |
| Healthy Environments and Consumer Safety Healthy Environments and Consumer Safety Branch | 10.6 | 12.0 | 15.4 | 15.4 | 15.4 | 12.5 |
| Pest Control Product Regulation Pest Management Regulatory Agency | 6.1 | 5.9 | 7.0 | 7.0 | 7.0 | 7.4 |
| First Nations and Inuit Health First Nations and Inuit Health Branch | 4.0 | 3.4 | 5.5 | 5.5 | 5.5 | 3.2 |
| Total Respendable Revenues | 55.8 | 58.9 | 69.1 | 69.1 | 69.1 | 63.8 |
Sources of Respendable and Non-Respendable Revenue continued...
(millions of dollars)
| Non-Respendable Revenues Main Classification and Source | Actual Revenues 2004-2005 | Actual Revenues 2005-2006 | 2006-2007 | |||
|---|---|---|---|---|---|---|
| Main Estimates | Planned Revenue | Total Authorities | Actual Revenues | |||
| Non-tax revenue: | ||||||
| Refunds of expenditures | 41.8 | 10.0 | 40.2 | |||
| Sales of goods and services | 2.5 | 2.6 | 3.5 | |||
| Other fees and charges | 6.8 | 7.0 | 8.9 | 8.9 | 7.9 | |
| Proceeds from the disposal of Crown assets | 0.2 | 0.2 | 0.2 | |||
| Miscellaneous non-tax revenues | 0.0 | 0.0 | 0.0 | |||
| Total Non-Respendable Revenue | 51.3 | 19.8 | 0.0 | 8.9 | 8.9 | 51.8 |
| Total Revenues | 107.1 | 78.7 | 69.1 | 78.0 | 78.0 | 115.6 |
| This table excludes amounts related to the Public Health Agency of Canada (PHAC). | ||||||
Table 6: Resource Requirements by Branch
Comparison of Main Estimates, 2006-2007 (RPP) planned spending and total authorities to actual spending by organization and program activity.
(millions of dollars)
| PROGRAM ACTIVITY | ||||||
|---|---|---|---|---|---|---|
| ORGANIZATION | Health Policy, Planning and Information | Health Products and Food | Healthy Environments and Consumer Safety | Pest Control Product Regulation | First Nations and Inuit Health | Total |
| Health Policy | ||||||
| (Main Estimates) | 269.7 | 267.9 | ||||
| (Planned Spending) | 269.6 | 296.6 | ||||
| (Total Authorities) | 275.2 | 275.2 | ||||
| (Actual Spending) | 253.8 | 253.8 | ||||
| Health Products and Food | ||||||
| (Main Estimates) | 204.2 | 204.2 | ||||
| (Planned Spending) | 204.2 | 204.2 | ||||
| (Total Authorities) | 225.6 | 225.6 | ||||
| (Actual Spending) | 212.1 | 212.1 | ||||
| Healthy Environments and Consumer Safety | ||||||
| (Main Estimates) | 238.2 | 238.2 | ||||
| (Planned Spending) | 237.5 | 237.5 | ||||
| (Total Authorities) | 243.6 | 243.6 | ||||
| (Actual Spending) | 234.6 | 234.6 | ||||
| Pest Management Regulatory Agency | ||||||
| (Main Estimates) | 40.2 | 40.2 | ||||
| (Planned Spending) | 40.1 | 40.1 | ||||
| (Total Authorities) | 45.1 | 45.1 | ||||
| (Actual Spending) | 40.3 | 40.3 | ||||
| First Nations and Inuit Health | ||||||
| (Main Estimates) | 1,961.0 | 1,961.0 | ||||
| (Planned Spending) | 1,997.9 | 1,997.9 | ||||
| (Total Authorities) | 1,956.2 | 1,956.2 | ||||
| (Actual Spending) | 1,922.2 | 1,992.2 | ||||
Resource Requirements by Branch continued...
(millions of dollars)
| PROGRAM ACTIVITY | ||||||
|---|---|---|---|---|---|---|
| ORGANISATION | Health Policy, Planning and Information | Health Products and Food | Healthy Environments and Consumer Safety | Pest Control Product Regulation | First Nations and Inuit Health | Total |
| Chief Financial Officer | ||||||
| (Main Estimates) | 3.2 | 9.1 | 8.5 | 2.0 | 16.5 | 39.3 |
| (Planned Spending) | 3.2 | 9.2 | 8.6 | 2.0 | 16.7 | 39.7 |
| (Total Authorities) | 5.5 | 5.7 | 7.9 | 3.4 | 17.9 | 40.4 |
| (Actual Spending) | 5.2 | 5.1 | 7.3 | 3.3 | 16.6 | 37.5 |
| Corporate Services | ||||||
| (Main Estimates) | 7.9 | 24.5 | 22.1 | 4.8 | 41.6 | 100.9 |
| (Planned Spending) | 7.9 | 24.3 | 21.9 | 4.8 | 41.3 | 100.2 |
| (Total Authorities) | 17.6 | 27.7 | 30.9 | 10.7 | 64.7 | 151.6 |
| (Actual Spending) | 17.5 | 27.4 | 30.6 | 10.6 | 64.2 | 150.3 |
| Departmental Executive | ||||||
| (Main Estimates) | 1.9 | 6.1 | 5.5 | 1.1 | 15.3 | 29.9 |
| (Planned Spending) | 1.8 | 6.1 | 5.6 | 1.1 | 15.3 | 29.9 |
| (Total Authorities) | 2.4 | 1.8 | 3.0 | 1.5 | 6.3 | 15.0 |
| (Actual Spending) | 2.3 | 1.1 | 2.4 | 1.4 | 5.5 | 12.7 |
| Public Affairs, Consultation and Regions | ||||||
| (Main Estimates) | 5.9 | 18.1 | 16.4 | 3.6 | 48.0 | 92.0 |
| (Planned Spending) | 5.9 | 18.1 | 16.3 | 3.6 | 47.9 | 91.8 |
| (Total Authorities) | 11.9 | 17.4 | 19.9 | 7.3 | 80.9 | 137.4 |
| (Actual Spending) | 11.6 | 16.6 | 19.2 | 7.1 | 79.5 | 134.0 |
| Total | ||||||
| (Main Estimates) | 288.6 | 262.0 | 290.7 | 51.7 | 2,082.4 | 2,975.4 |
| (Planned Spending) | 288.4 | 262.1 | 289.9 | 51.6 | 2,119.1 | 3,011.1 |
| (Total Authorities) | 312.6 | 278.2 | 305.3 | 68.0 | 2,126.0 | 3,090.1 |
| (Actual Spending) | 290.4 | 262.3 | 294.1 | 62.7 | 2,088.0 | 2,997.5 |
| % of Total | 9.7% | 8.8% | 9.8% | 2.1% | 69.7% | 100.0% |
Table 7a: User Fees Act
|
Health Products and Foods Branch (HPFB) |
2006-07 |
Planning Years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. User Fees |
Fee Type |
Fee-setting authority |
Date Last Modified |
Forecast Revenue ($000) |
Actual Revenue ($000) |
Full Cost ($000) |
Performance Standard |
Performance Results |
Fiscal Year |
Forecast Revenue ($000) |
Estimated Full Cost ($000) |
|
|
Authority to Sell Drugs Fees |
Regulatory (R) |
Financial Administration Act (FAA) |
Dec.1994 |
8,039 |
8,020 |
27,599 |
120 calendar days to update the Drug Product Database following notification |
96% within 120 calendar days |
2007-08 2008-09 2009-10 |
8,032 18,500 18,962 |
36,900 37,822 38,768 |
|
|
Certificates of Pharmaceutical Product (Drug Export) Fees |
Other (O) |
Ministerial authority to enter into contract |
May 2000 |
120 |
117 |
386 |
5 working days to issue certificate |
95% certificates issued within 5 working days |
2007-08 2008-09 2009-10 |
117 157 161 |
157 161 165
|
|
|
Drug Establishment Licensing Fees |
R |
FAA |
Dec. 1997 |
5,141 |
6,004 |
8,368 |
250 calendar days to issue / renew licence |
90% licences issued/renewed within 300 calendar days |
2007-08 2008-09 2009-10 |
6,014 13,900 14,247 |
13,900 14,247 14,603 |
|
|
Drug Master File Fees |
O |
Ministerial authority to enter into contract |
Jan. 1996 |
150 |
132 |
219 |
30 calendar days |
100% within 30 calendar days |
2007-08 2008-09 2009-10 |
133 378 387 |
378 387 397 |
|
|
Drug Submission Evaluation Fees |
R |
FAA |
Aug. 1995 |
18,693 |
21,239 |
76,129 |
Review time to first decision (calendar days) |
Average review time to first decision (calendar days) |
2007-08 2008-09 2009-10 |
21,273 49,600 50,840 |
66,200 67,855 69,551 |
|
|
Pharmaceuticals
|
|
|
|
|
|
|
NDS: Priority NAS = 180 |
193 |
|
|
|
|
|
NDS: NOC-C NAS = 200 |
184 |
|||||||||||
|
NDS: NOC-C Clin / C&M = 200 |
200 |
|||||||||||
|
NDS: NAS = 300 |
251 |
|||||||||||
|
NDS: Clin/C&M=300 |
252 |
|||||||||||
|
NDS: Clin only = 300 |
188 |
|||||||||||
|
NDS: Comp / C&M = 300 |
230 |
|||||||||||
|
ANDS: C&M/Labelling = 180 |
165 |
|||||||||||
|
ANDS: Comp/C&M = 180 |
165 |
|||||||||||
|
SNDS: Priority Clin Only = 180 |
156 |
|||||||||||
|
SNDS: Priority Clin / C&M = 180 |
131 |
|||||||||||
|
SNDS: NOC-c Clin/ C&M = 200 |
199 |
|||||||||||
|
SNDS: Clin/C&M = 300 |
281 |
|||||||||||
|
SNDS: Clin only = 300 |
250 |
|||||||||||
|
SNDS: Comp/C&M = 180 |
224 |
|||||||||||
|
SNDS: C&M/ Labelling = 180 |
176 |
|||||||||||
|
SNDS: Rx to OTC (switch) – no new indication = 180 |
142 |
|||||||||||
|
SNDS: Labelling only = 60 |
39 |
|||||||||||
|
SANDS: Comp / C&M = 180 |
180 |
|||||||||||
|
SANDS: C&M / Labelling = 180 |
168 |
|||||||||||
|
SANDS: Labelling only = 60 |
58 |
|||||||||||
|
DINA with data = 210 |
190 |
|||||||||||
|
DINA form only = 180 |
149 |
|||||||||||
|
DIND with data = 210 |
117 |
|||||||||||
|
DIND form only = 180 |
138 |
|||||||||||
|
Biological Products
|
|
|
|
|
|
|
NDS: Priority NAS = 180 |
274 |
|
|
|
|
|
NDS: Priority Clin/C&M = 180 |
378 |
|||||||||||
|
NDS: NOC-C Clin / C&M = 200 |
185 |
|||||||||||
|
NDS: NAS = 300 |
560 |
|||||||||||
|
NDS: Clin/C&M=300 |
481 |
|||||||||||
|
SNDS: Priority Clin Only = 180 |
175 |
|||||||||||
|
SNDS: Clin/C&M = 300 |
381 |
|||||||||||
|
SNDS: Clin only = 300 |
408 |
|||||||||||
|
SNDS: Comp/C&M = 180 |
379 |
|||||||||||
|
SNDS: C&M/ Labelling = 180 |
188 |
|||||||||||
|
DINB with data = 210 |
130 |
|||||||||||
|
DINB form only = 180 |
10 |
|||||||||||
|
Medical Device Licence Application Fees |
R |
FAA |
Aug.1998 |
3,352 |
3,443 |
12,348 |
Time to first decision (calendar days) |
Time to first decision (calendar days) |
2007-08 2008-09 2009-10 |
3,449 7,200 7,380 |
9,600 9,840 10,086 |
|
|
Class II = 15 (process) |
13 |
|||||||||||
|
Class II amendment = 15 |
11 |
|||||||||||
|
Class III = 75 |
52 |
|||||||||||
|
Class III amendment = 75 |
38 |
|||||||||||
|
Class IV = 90 |
62 |
|||||||||||
|
Class IV amendment = 90 |
49 |
|||||||||||
|
Fees for Right to Sell a Licensed Medical Device |
R |
FAA |
Aug. 1998 |
1,730 |
1,790 |
9,437 |
20 calendar days from deadline for receipt of annual notification to update the Medical Devices Active License Listing (MDALL) database |
100% within 20 calendar days |
2007-08 2008-09 2009-10 |
1,793 6,300 6,457 |
12,700 13,017 13,342 |
|
|
Medical Device Establishment Licensing Fees |
R |
FAA |
Jan 2000 |
2,163 |
2,104 |
4,037 |
120 calendar days to issue / renew licence |
90% licences issued/renewed within 120 calendar days |
2007-08 2008-09 2009-10 |
2,107 13,900 14,247 |
13,900 14,247 14,603 |
|
|
Veterinary Drug Evaluation Fees |
R |
FAA |
Mar. 1996 |
776 |
769 |
6,859 |
Review time to first decision (calendar days) |
Average review time to first decision (calendar days) |
2007-08 2008-09 2009-10 |
770 789 809
|
6, 480 6,642 6,808
|
|
|
NDS = 300 |
671 |
|||||||||||
|
SNDS = 240 |
539 |
|||||||||||
|
SABNDS = 240 |
295 |
|||||||||||
|
Admin = 90 |
134 |
|||||||||||
|
DIN = 120 |
195 |
|||||||||||
|
NC = 90 |
177 |
|||||||||||
|
IND/ESC = 60 |
58 |
|||||||||||
|
Labels = 45 |
28 |
|||||||||||
|
Emergency Drug Release = 2 |
90+% within 2 days |
|||||||||||
|
Subtotal (R) |
|
|
|
39,894 |
43,369 |
144,778 |
|
|
2007-08 2008-09 2009-10 |
43,438 110,189 112,937 |
159,680 163,670 167,761 |
|
|
Subtotal (O) |
270 |
249 |
605 |
2007-08 2008-09 2009-10 |
250 535 548 |
535 548 562 |
||||||
|
Total |
40,164 |
43,618 |
145,383 |
2007-08 2008-09 2009-10 |
43,688 110,724 113,485 |
160,215 164,218 168,323 |
||||||
|
Acronyms NDS: New Drug Submission SNDS: Supplemental New Drug Submission ANDS/ABNDS: Abbreviated New Drug Submission SANDS/SABNDS: Supplemental Abbreviated New Drug Submission DIN: Drug Identification Number Application INDS: Investigational New Drug Submission ESC: Experimental Studies Certificate NC: Notifiable Change NAS: New Active Substance OTC: Over the Counter Rx: Prescription Clin: Clinuical Comp: Comparative Bio, Clinical or Pharmacodynamic C&M: Chemistry and Manufacturing NOC-C: Notice of Compliance with Conditions |
Detailed performance targets Forecast and actual revenue are reported on a modified cash accounting basis. Costing information was developed using the Program Activity Architecture coding structure as directed through Treasury Board. Under the Cost Recovery Initiative, HPFB is in the process of implementing a revised cost recovery framework, including revised fees and service standards; target for implementation is 2008-2009. |
|||||||||||
|
Pest Management Regulatory Agency (PMRA) |
2006–07 |
Planning Years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. User Fee |
Fee Type |
Fee-setting |
Date Last |
Forecast Revenue |
Actual Revenue |
Full Cost |
Performance |
Performance Results |
Fiscal Year |
Forecast Revenue |
Estimated Full Cost |
|
Fees to be paid for Pest Control Product Application Examination Service |
Regulatory (R) |
Pest Control Products Act (PCPA) |
April 1997 |
2,637 |
3,825 |
32,246 |
Target is 90% of submissions in all categories to be processed within time shown. Category A Category B Category C Category D Category E *Includes deviations from Management of Submission Policy |
Category A = 94% Category B = 94% Category C = 86% Category D (Minor Use only) = 79% Category E = 50% |
2007-08 2008-09 2009-10 |
8,000 8,000 8,000 |
58,200 54,100 56,900 |
|
Fees to be paid for the right or privilege to manufacture or sell a pest control product in Canada and for establishing a Maximum Residue Limit in relation to a pest control product. |
R |
Financial Adminis-
|
April 1997 |
5,353 |
4674.45 |
37,854 |
All stakeholders have been consulted on the proposed service standard for invoicing clients
|
No objection and based on that, 100% of all fees for the right or privilege to manufacture or sell a pest control product in Canada have been invoiced by April 30th of the fiscal year |
|
|
|
|
|
|
|
|
TOTAL 7,990 |
TOTAL 8,499
|
TOTAL 70,100 |
|
|
|
Sub-Total 2007–08 $8,000 Sub-Total 2008–09 $8,000 Sub-Total 2009–10 $8,000 TOTAL: 24,000 |
2007–08 $58,200 2008–09 $54,100 2009-10 $56,900 TOTAL: 169,200 |
| Corporate Services Branch |
2006-07 |
Planning Years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| User Fee | Fee Type | Fee Setting Authority | Date Last Modified |
Forecast Revenue ($000) |
Actual Revenue ($000) |
Full Cost ($000) |
Performance Standard |
Performance Results1 |
Fiscal Year |
Forecast Revenue ($000) |
Estimated Full Cost ($000) |
|
Fees charged for the processing of access requests filed under the Access to Information Act (ATIA) |
Other products and services (O) |
Access to Information Act |
1992 |
$20.93 |
$12.1 |
$1,366
|
Response provided within 30 days following receipt of request; the response time may be extended pursuant to section 9 of the ATIA. Notice of extension to be sent within 30 days after receipt of request. The Access to Information Act provides fuller details
|
Of the 2,017 requests, 1,643 (81.5%) requests were completed during the 2006–2007 reporting period.
The Department was able to respond within 30 days or less in 626 (38.1%) of completed cases. Response times for the remaining cases were 280 (17.0%) within 31 days to 60 days, 400 (24.3%) within 61 to 120 days, and 337 (20.5%) in 121 or more days. |
2007-08 2008-09 2009-10 |
$13.00 $13.00 $13.00 See note 2 under Section C – Other Information |
$1,400 $1,400 $1,400 Section Note3 under Section C – Other Information |
|
Sub-Total (R) Sub-Total (O) |
|
|
|
|
$0 $12.1 |
$0 $1,366 |
|
|
2007-08 |
$13.00 $13.00 $13.00 |
$1,400 $1,400 $1,400 |
| Total | $39.00 | $4,200 | |||||||||
| Date Last Modified: NA | |||||||||||
| 1. Projection based on actual revenue received during FY 2006-07.Due to the nature and varying complexity of ATI requests, it is unknown what fees may be applicable until a request is processed. Under certain circumstances, fees may be waived. 2. Estimated direct cost associated with ATI requests. |
|||||||||||
Table 7b, 8 and 9
For detailed information on Policy on Service Standards for External Fees (Table 7b), Major Regulatory Initiatives (Table 8) and Details on Project Spending (Table 9), please visit the following website: http://www.tbs-sct.gc.ca/rma/dpr2/06-07/index_e.asp.
Table 10a: Summary of Transfer Payments by Program Activity
This table reflects the break down of Transfer Payments (Grants and Contributions) by Program Activity. For more details refer to Table 10(b).
(millions of dollars)
| Program Activities | 2004-2005 | 2005-2006 | 2006-2007 | |||
|---|---|---|---|---|---|---|
| Actual Spending | Actual Spending | Main Estimates | Planned Spending | Total Authorities | Actual Spending | |
|
Grants
|
||||||
| Health Policy, Planning and Information | 50.7 | 54.6 | 57.1 | 57.1 | 53.2 | 43.3 |
| Health Products and Foods | 5.4 | 5.5 | 5.9 | 5.9 | 5.7 | 5.5 |
| Healthy Environments and Consumer Safety | 1.5 | 1.2 | 5.1 | 5.1 | 5.1 | 4.5 |
| First Nations and Inuit Health | 0.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| TOTAL GRANTS | 57.6 | 91.3 | 98.1 | 98.1 | 94.0 | 83.3 |
|
Contributions
|
||||||
| Health Policy, Planning and Information | 222.8 | 236.3 | 135.9 | 135.9 | 134.3 | 129.5 |
| Health Products and Foods | 0.4 | 4.1 | 4.0 | 4.0 | 3.8 | 3.8 |
| Healthy Environments and Consumer Safety | 35.8 | 42.3 | 39.6 | 39.7 | 39.0 | 38.7 |
| First Nations and Inuit Health | 858.9 | 826.8 | 911.7 | 918.5 | 907.2 | 896.6 |
| TOTAL CONTRIBUTIONS | 1,118.0 | 1,109.5 | 1,091.2 | 1,098.1 | 1,084.3 | 1,068.6 |
| TOTAL TRANSFER PAYMENTS | 1,175.6 | 1,200.8 | 1,189.3 | 1,196.2 | 1,178.3 | 1,151.9 |
|
This table excludes amounts related to the Public Health Agency of Canada (PHAC). The increase in First Nations and Inuit Health Grant expenditures is due to funding for the Territorial Health Access Fund and Operational Secretariat and the Territorial Medical Travel Fund. The decrease in Health Policy, Planning and Information contributions is largely due to the reduction for the Primary Health Care Transition Fund. |
||||||
Table 10b: Details on Transfer Payment Programs (TPPs)
HEALTH POLICY, PLANNING AND INFORMATION
- Grant to Health Council of Canada
- Grant to Canadian Agency for Drugs and Technologies in Health
- Grant to Canadian Patient Safety Institute
- Contributions Program to improve access to health services for official language minority communities
- Health Care Strategies and Policy Contribution Program
- Contributions for the Primary Health Care Transition Fund
HEALTH PRODUCTS AND FOOD
- Grant to Canadian Blood Services: Blood Safety and Effectiveness and Research and Development
HEALTHY ENVIRONMENTS AND CONSUMER SAFETY
- Alcohol and Drug Treatment and Rehabilitation Contribution Program
- Drug Strategy Community Initiatives Fund
- Contributions in support of the Federal Tobacco Control Strategy
FIRST NATIONS AND INUIT HEALTH
- Nunavut Medical Travel Fund
- Grant to Government ofYukon for theTerritorial Health
Access Fund and Operational Secretariat - Contributions for First Nations and Inuit Health Benefits
- Payments to Aboriginal Health Institute/Centre for advancement of Aboriginal peoples' health
- Contributions for First Nations and Inuit Health Facilities and Capital Program
- Contributions for First Nations and Inuit Community Programs
- Payments to Indian bands, associations or groups for control and provision of health services
- Contributions for First Nations and Inuit Health Governance and Infrastructure Support
- Contributions for Bigstone Non-Insured Health Benefits Pilot Project
- Contributions for First Nations and Inuit Health Protection
- Contributions for First Nations and Inuit Primary Health Care
Table 11: Conditional Grants (Foundations)
Canadian Health Services Research Foundation (CHSRF)
Canada Health Infoway Inc. ( Infoway )
Canadian Institute for Health Information (CIHI)
Supplementary information on Conditional Grants (Foundations)
Table 12: Financial Statements
Statement of Management Responsibility
Responsibility for the integrity and objectivity of the accompanying financial statements for the year ended March 31, 2007 and all information contained in these statements rests with Health Canada's management. These financial statements have been prepared by management in accordance with accounting standards issued by the Treasury Board of Canada Secretariat which are consistent with Canadian generally accepted accounting principles for the public sector.
Management is responsible for the integrity and objectivity of the information in these financial statements. Some of the information in the financial statements is based on management’s best estimates and judgment and gives due consideration to materiality. To fulfil its accounting and reporting responsibilities, management maintains a set of accounts that provides a centralized record of Health Canada’s financial transactions. Financial information submitted to the Public Accounts of Canada and included in Health Canada's Departmental Performance Report is consistent with these financial statements.
Management maintains a system of financial management and internal control designed to provide reasonable assurance that financial information is reliable, that assets are safeguarded and that transactions are in accordance with the Financial Administration Act, are executed in accordance with prescribed regulations, within Parliamentary authorities, and are properly recorded to maintain accountability of Government funds.
Management also seeks to ensure the objectivity and integrity of data in its financial statements by careful selection, training and development of qualified staff, by organizational arrangements that provide appropriate divisions of responsibility, and by communication programs aimed at ensuring that regulations, policies, standards and managerial authorities are understood throughout Health Canada.
Management is supported by the Departmental Audit and Evaluation Committee, which provides assurance on risk management strategy and practices, management control frameworks and practices, policy and program effectiveness and improvement, and other information used for decision-making and reporting. The Committee oversees internal audit and evaluation activities and approves the department audit and evaluation plan. It also reviews the results of audits and evaluations as well as management responses and action plans developed to address related recommendations. The Committee membership is comprised of the Deputy Minister or the Associate Deputy Minister, the Assistant Deputy Ministers or their alternates, a Regional Director General, a representative from the Audit and Accountability Bureau and the Director of the Evaluation Division. A representative from the Office of the Auditor General participates as an observer. The financial statements of Health Canada have not been audited.
Suzanne Vinet
Acting Deputy Minister
Ottawa, Canada
Date: August 9, 2007
Marcel Nouvet
Interim Chief Financial Officer
Ottawa, Canada
Date: August 9, 2007
Statement of Operations (unaudited)
For the year ended March 31
(in thousands of dollars)
| 2007 | 2006 | ||||||
|---|---|---|---|---|---|---|---|
| Expenses | First Nations and Inuit Health | Health Policy, Planning and Information | Health Products and Food | Healthy Environments and Consumer Safety | Pest Control Product Regulation | Total | Total |
| Transfer payments | 909,281 | 594,917 | 9,198 | 42,245 | - | 1,555,641 | 1,191,194 |
| Contingent Liability Expenses | (2,504) | 1,023,476 | - | (20) | (150) | 1,020,802 | (12) |
| Salaries and wages | 272,447 | 57,700 | 243,436 | 178,198 | 53,405 | 805,186 | 783,529 |
| Utilities, material and supplies | 412,826 | 2,492 | 16,457 | 17,677 | 2,513 | 451,965 | 401,801 |
| Professional and special services | 294,187 | 42,168 | 40,259 | 47,432 | 6,926 | 430,972 | 387,867 |
| Travel- Non-Insured Health Patient | 122,676 | - | - | - | - | 122,676 | 112,713 |
| Accommodation | 19,347 | 3,735 | 14,964 | 10,756 | 3,421 | 52,223 | 50,198 |
| Purchased repair and maintenance | 14,705 | 2,965 | 9,271 | 9,101 | 2,028 | 38,070 | 42,570 |
| Travel and relocation | 20,654 | 2,816 | 5,235 | 7,602 | 830 | 37,137 | 38,748 |
| Information | 8,529 | 1,951 | 4,574 | 10,913 | 1,017 | 26,984 | 14,716 |
| Communications | 10,983 | 1,330 | 4,668 | 4,943 | 849 | 22,773 | 18,442 |
| Amortization | 7,136 | 3 | 6,967 | 6,754 | 274 | 21,134 | 22,492 |
| Rentals | 1,521 | 933 | 869 | 857 | 197 | 4,377 | 4,356 |
| Bad debts | 249 | 54 | 143 | 144 | 33 | 623 | - |
| Other | (181) | 43 | 189 | 278 | 19 | 348 | 4,216 |
| 2,091,856 | 1,734,583 | 356,230 | 336,880 | 71,362 | 4,590,911 | 3,072,830 | |
| Revenues | |||||||
| Sales of goods and services | |||||||
| Services of a regulatory nature | 19 | - | 22,324 | 47 | 3,364 | 25,754 | 21,364 |
| Rights and privileges | 21 | - | 17,123 | 52 | 4,112 | 21,308 | 21,206 |
| Services of a non-regulatory nature | 4,446 | - | 365 | 12,368 | 53 | 17,232 | 16,946 |
| Lease and Use of Public Property | 416 | - | 2 | 4 | 1 | 423 | 448 |
| Revenues from fines | - | - | - | 2,348 | - | 2,348 | 2,759 |
| Interest | 127 | - | 573 | 318 | 255 | 1,273 | 320 |
| Other | 1,809 | 5 | 3,200 | 1,745 | 1,258 | 8,017 | 7,245 |
| 6,838 | 5 | 43,587 | 16,882 | 9,043 | 76,355 | 70,288 | |
| Net cost of operations | 2,085,018 | 1,734,578 | 312,643 | 319,998 | 62,319 | 4,514,556 | 3,002,542 |
| The accompanying notes are an integral part of the financial statementss | |||||||
Statement of Financial Position (unaudited)
As at March 31
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Assets | ||
| Financial assets | ||
| Accounts receivable and advances (Note 4) | 33,472 | 27,360 |
| 33,472 | 27,360 | |
| Non-financial assets | ||
| Prepaid expenses | 2 | - |
| Tangible capital assets (Note 5) | 108,116 | 109,824 |
| 108,118 | 109,824 | |
| 141,590 | 137,184 | |
| Liabilities and Equity of Canada | ||
| Liabilities | ||
| Accounts payable and accrued liabilities | 395,377 | 402,718 |
| Vacation pay and compensatory leave | 39,055 | 37,205 |
| Deferred revenue | 3,683 | 4,944 |
| Employee severance benefits (Note 6) | 134,294 | 122,332 |
| Other liabilities (Note 7) | 1,461,712 | 10,684 |
| 2,034,121 | 577,883 | |
| Equity of Canada | (1,892,531) | (440,699) |
| 141,590 | 137,184 | |
| Contingent Liabilities (Note 8) | ||
| Contractual Obligations (Note 9) | ||
| The accompanying notes are an integral part of the financial statements | ||
Statement of Equity (unaudited)
For the year ended March 31
| (in thousands of dollars) | 2007 | 2006 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equity of Canada, beginning of year | (440,699) | (615,016) | |||||||||||||||||||||||||
| Net cost of operations | (4,514,556) | (3,002,542) | |||||||||||||||||||||||||
| Current year appropriations used (Note 3) | 2,997,550 | 2,891,980 | |||||||||||||||||||||||||
| Revenue not available for spending | (12,597) | (11,234) | |||||||||||||||||||||||||
| Change in net position in the Consolidated Revenue Fund (Note 3) | (14,173) | 210,538 | |||||||||||||||||||||||||
| Services provided without charge by other government departments (Note 10) | 91,944 | 85,575 | |||||||||||||||||||||||||
| Equity of Canada, end of year | (1,892,531) | (440,699) | |||||||||||||||||||||||||
| The accompanying notes are an integral part of the financial statements | |||||||||||||||||||||||||||
Statement of Cash Flow (unaudited)
For the year ended March 31
| (in thousands of dollars) | 2007 | 2006 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Operating transactions | ||||||||||||||||||||||||
| Net cost of operations | 4,514,556 | 3,002,542 | ||||||||||||||||||||||
| Non-cash items: | ||||||||||||||||||||||||
| Amortization of tangible capital assets (Note 5) | (21,134) | (22,492) | ||||||||||||||||||||||
| Gain (loss) on disposal of capital and non-capital assets | (31) | 1,003 | ||||||||||||||||||||||
| Services provided without charge by other government departments (Note 10) | (91,944) | (85,575) | ||||||||||||||||||||||
| Variations in Statement of Financial Position: | ||||||||||||||||||||||||
| Increase (decrease) in accounts receivable, advances and prepaids | 6,114 | (27,964) | ||||||||||||||||||||||
| Decrease (increase) in liabilities | (1,456,238) | 215,995 | ||||||||||||||||||||||
| Cash used by Operating Activities | 2,951,323 | 3,083,509 | ||||||||||||||||||||||
| Capital investment activities | ||||||||||||||||||||||||
| Acquisitions of tangible capital assets (Note 5) | 19,542 | 7,894 | ||||||||||||||||||||||
| Proceeds on disposal of tangible capital assets | (85) | (119) | ||||||||||||||||||||||
| Cash used by Investment Activities | 19,457 | 7,775 | ||||||||||||||||||||||
| Financing Activities | ||||||||||||||||||||||||
| Net cash provided by Government of Canada | (2,970,780) | (3,091,284) | ||||||||||||||||||||||
| Cash used by Financing Activities | (2,970,780) | (3,091,284) | ||||||||||||||||||||||
| The accompanying notes are an integral part of the financial statements | ||||||||||||||||||||||||
Notes to the Financial Statements (unaudited)
1. Authority and purpose
The Department of Health was established effective July 12, 1996 under the Department of Health Act to participate in the promotion and preservation of the health of the people of Canada. It is named in Schedule I of the Financial Administration Act and reports through the Minister of Health. Priorities and reporting are aligned under the following program activities:
First Nations and Inuit Health
The First Nations and Inuit Health program activity objectives include improving health outcomes; ensuring availability of, and access to, quality health services; and supporting greater control of the health system by First Nations and Inuit. Together with First Nations and Inuit, the First Nations and Inuit Health Branch through its regional offices, delivers public health and community health programs on-reserve, these include environmental health and communicable and non-communicable disease prevention, and provision of primary health care services through nursing stations and community health centres in remote and/or isolated communities to supplement and support the services that provincial, territorial and regional health authorities provide. The First Nations and Inuit Health program activity also supports targeted health promotion programs for Aboriginal people, regardless of residency (e.g. Aboriginal Diabetes Initiative) as well as counselling, addictions and mental wellness services. The Non-Insured Health Benefits coverage of drug, dental care, vision care, medical supplies and equipment, short-term crisis intervention mental health services, and medical transportation is available to all registered Indians and recognized Inuit in Canada, regardless of residency.
Health Policy, Planning and Information
The Health Policy, Planning and Information program activity provides advice and support to the Minister, the departmental executives and to program branches in the areas of policy development, intergovernmental and international affairs, strategic planning, program delivery and review and the administration of the Canada Health Act . It also contributes to improved health outcomes for Canadians by promoting the increased and more effective use of information and communications technologies; by improving access to reliable health information; by providing policy research and analysis to support evidence-based decision-making; by working with official language minority communities and others to improve access to health services in the official language of choice; and by taking into account Canadians' privacy expectations with respect to health information.
Health Products and Food
Health Canada is responsible for a broad range of health protection and promotion activities that affect the everyday lives of Canadians. As the federal authority responsible for the regulation of health products and food, Health Products and Food Branch evaluates and monitors the safety, quality and effectiveness of thousands of drugs (human and veterinary), vaccines, blood and blood products, biologics and genetic therapies, medical devices and natural health products, as well as the safety of the foods Canadians eat. It also provides useful information about risks and benefits related to health products and food so that Canadians can make informed decisions about their health and well-being. Ongoing regulatory responsibilities span the life cycle of health products and food, from clinical trials to surveillance, compliance and enforcement. The branch is also facing challenges associated with rapid advances in technology and scientific breakthroughs that have resulted in the growth of an unprecedented number of biologics, genetic therapies and vaccines and genetically modified and other novel foods. These challenges are met by drawing on sound science and effective risk management in evidence-based decision-making. These disciplines are integrated into daily operations, and together with the branch health promotion activities, they enable timely access to safe and effective health products and food for Canadians.
Healthy Environments and Consumer Safety
Under this Program Activity, Health Canada addresses many elements of day-to-day living that have an impact on the health of Canadians. These include drinking water safety, air quality, radiation exposure, substance use and abuse (including alcohol), consumer product safety, tobacco and second hand smoke, workplace health, and chemicals in the workplace and in the environment. Health Canada is also engaged in other health and safety related activities, including the Government's public safety and anti-terrorism initiatives, inspection of food and potable water for the travelling public, and health contingency planning for visiting foreign dignitaries. The broad national mandate flows from legislation including the Food and Drugs Act, the Controlled Drugs and Substances Act, the Hazardous Products Act, the Radiation Emitting Devices Act, the Canadian Environmental Protection Act, the Tobacco Act and others. Results are delivered through partnerships and by an active presence throughout every region of the country.
Pest Control Product Regulation
To help prevent unacceptable risks to people and the environment, Health Canada regulates the importation, sale and use of pesticides under the federal authority of the Pest Control Products Act (PCPA ) and Regulations. The scope of work is extensive with more than 5,000 registered pesticides - including herbicides, insecticides, fungicides, antimicrobial agents, pool chemicals, microbials, material and wood preservatives, animal and insect repellents as well as insect and rodent-controlling devices. Ongoing regulatory responsibilities constitute the majority of the work under this program activity. Using internationally accepted approaches and protocols; Health Canada conducts science-based health, environmental and value assessments. Pesticides are registered only if the health and environmental risks are considered acceptable, and if the product is effective. Health Canada sets maximum pesticide residue limits for food commodities under the Food and Drugs Act . Older pesticides are re-evaluated to determine if their use continues to be acceptable under current scientific approaches. Health Canada facilitates, encourages and maximizes compliance with the PCPA and the conditions of registration and also develops and promotes the use of sustainable pest management practices and products in cooperation with stakeholders.
The Department is responsible for the administration and enforcement of the following statutes and/or regulations, for which the Minister of Health is responsible for the Department and remains accountable to Parliament: Canada Health Act, Canadian Centre on Substance Abuse Act, Canadian Environmental Protection Act, Controlled Drugs and Substance Act, Department of Health Act, Fitness and Amateur Sport Act, Food and Drugs Act, Hazardous Materials Information Review Act, Hazardous Products Act, Patent Act, Pest Control Products Act, Pesticide Residue Compensation Act, Quarantine Act, Queen Elizabeth II Canadian Research Fund Act, Radiation Emitting Devices Act, Tobacco Act, and the Human Assisted Reproduction Act.
2. Significant accounting policies
The financial statements have been prepared in accordance with Treasury Board accounting policies which are consistent with Canadian generally accepted accounting principles for the public sector. Significant accounting policies are as follows:
(a) Parliamentary appropriations
The Department of Health is financed by the Government of Canada through Parliamentary appropriations. Appropriations provided to the department do not parallel financial reporting according to generally accepted accounting principles since appropriations are primarily based on cash flow requirements. Consequently, items recognized in the statement of operations and the statement of financial position are not necessarily the same as those provided through appropriations from Parliament. Note 3 provides a high-level reconciliation between the two bases of reporting.
(b) Net Cash Provided by Government
The department operates within the Consolidated Revenue Fund (CRF). The CRF is administered by the Receiver General for Canada. All cash received by the department is deposited to the CRF and all cash disbursements made by the department are paid from the CRF. Net cash provided by Government is the difference between all cash receipts and all cash disbursements including transactions between departments of the federal government.
(c) Change in net position in the Consolidated Revenue Fund
The change in net position in the Consolidated Revenue Fund is the difference between the net cash provided by Government and appropriations used in a year, excluding the amount of non respendable revenue recorded by the department. It results from timing differences between when a transaction affects appropriations and when it is processed through the CRF.
(d) Revenues
Revenues are accounted for in the period in which the underlying transaction or event occurred that gave rise to the revenues. Types of revenues collected include medical devices, radiation dosimetry, drug submission evaluation, veterinary drugs, pest management regulation, product safety, hospital revenues resulting from payments for services provided to First Nations and Inuit Health hospitals, which are covered under provincial or territorial plans, and for the sale of drugs and health services for First Nations communities. Revenues that have been received but not yet earned are disclosed as deferred revenues.
(e) Expenses
Expenses are recorded on the accrual basis:
- Grants are recognized in the year in which the conditions for payment are met. In the case of grants which do not form part of an existing program, the expense is recognized when the Government announces a decision to make a non-recurring transfer, provided the enabling legislation or authorization for payment receives parliamentary approval prior to the completion of the financial statements;
- Contributions are recognized in the year in which the recipient has met the eligibility criteria or fulfilled the terms of a contractual transfer agreement;
- Vacation pay and compensatory leave are expensed as the benefits accrue to employees under their respective terms of employment.
- Services provided without charge by other government departments for accommodation, the employer’s contribution to the health and dental insurance plans, salary and associated expenditures of legal services and the worker's compensation coverage are recorded as operating expenses at their estimated cost.
(f) Accounts receivable
Accounts receivables are stated at amounts expected to be ultimately realized; a provision is made for receivables where recovery is considered uncertain.
(g) Employee future benefits
- Pension benefits: Eligible employees participate in the Public Service Pension Plan, a multiemployer plan administered by the Government of Canada. The department's contributions to the Plan are charged to expenses in the year incurred and represent the total departmental obligation to the Plan. Current legislation does not require the department to make contributions for any actuarial deficiencies of the Plan.
- Severance benefits: Employees are entitled to severance benefits under labour contracts or conditions of employment. These benefits are accrued as employees render the services necessary to earn them. The obligation relating to the benefits earned by employees is calculated using information derived from the results of the actuarially determined liability for employee severance benefits for the Government as a whole.
(h) Contingent liabilities
Contingent liabilities are potential liabilities which may become actual liabilities when one or more future events occur or fail to occur. To the extent that the future event is likely to occur or fail to occur, and a reasonable estimate of the loss can be made, an estimated liability is accrued and an expense recorded. If the likelihood is not determinable or an amount cannot be reasonably estimated, the contingency is disclosed in the notes to the financial statements.
(i) Environmental liabilities
Environmental liabilities reflect the estimated costs related to the management and remediation of environmentally contaminated sites. Based on management's best estimates, a liability is accrued and an expense recorded when the contamination occurs or when the department becomes aware of the contamination and is obligated, or is likely to be obligated to incur such costs. If the likelihood of the department's obligation to incur these costs is not determinable, or if an amount cannot be reasonably estimated, the costs are disclosed as contingent liabilities in the notes to the financial statements.
(j) Tangible Capital Assets
All tangible capital assets and leasehold improvements having an initial cost of $10,000 or more are recorded at their acquisition cost. Health Canada does not capitalize intangibles, works of art and historical treasures that have cultural, aesthetic or historical value, immovable assets located on Indian Reserves and museum collections.
Amortization of capital assets is done on a straight-line basis over the estimated useful life of the capital asset as follows:
| Asset class | Sub-asset class | Amortization Period |
|---|---|---|
| Buildings | Buildings | 25 years |
| Works and infrastructure | Works and infrastructure | 25 years |
| Leasehold improvements | Leasehold improvements | Lease term, max. 40 years |
| Machinery and equipment | Machinery and equipment | 8-12 years |
| Computer equipment | 3-5 years | |
| Computer software | 3 years | |
| Other equipment | 10-12 years | |
| Vehicles | Motor Vehicles | 4-7 years |
| Other Vehicles | 10 years |
(k) Prepaid expenses
Prepaid expenses include prepayments of operating expenses and transfer payments. Prepaid transfer payments consist of contributions advanced to recipients as of March 31 for which it is known that the costs will be incurred by the recipient in the subsequent fiscal year and the amount can be readily determined based on available information.
(l) Measurement uncertainty
The preparation of these financial statements in accordance with accounting policies issued by the Treasury Board of Canada which are consistent with Canadian generally accepted accounting principles for the public sector requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses reported in the financial statements. At the time of preparation of these statements, management believes the estimates and assumptions to be reasonable. The most significant items where estimates are used are contingent liabilities, environmental liabilities, the liability for employee severance benefits and the useful life of tangible capital assets. Actual results could differ from those estimated. Management’s estimates are reviewed periodically and, as adjustments become necessary, they are recorded in the financial statements in the year they become known.
3. Parliamentary appropriations
Health Canada receives most of its funding through annual Parliamentary appropriations. Items recognized in the statement of operations and the statement of financial position in one year may be funded through Parliamentary appropriations in prior, current or future years. Accordingly, the Department has different net cost of operations for the year on a government funding basis than on an accrual accounting basis. The differences between net cost of operations and appropriations are reconciled in the following tables.
(a) Reconciliation of net cost of operations to current year appropriations used:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Net cost of operations | 4,514,556 | 3,002,542 |
| Adjustments for items affecting net cost of operations but not affecting appropriations: | ||
| Add (Less): | ||
| Services provided without charge by other government departments | (91,944) | (85,575) |
| Amortization | (21,134) | (22,492) |
| Employee severance benefits | (11,961) | (21,856) |
| Refund/adjustment of previous year's expenditures | 40,390 | 17,224 |
| Revenue not available for spending | 12,597 | 11,234 |
| Allowance for Bad Debt | (623) | 0 |
| Justice Canada legal fees | (11,785) | (10,488) |
| Vacation pay and compensatory leave | (1,918) | (2,736) |
| Adjustments to previous years revenues | 0 | 0 |
| Adjustments to opening balances | 0 | 0 |
| Other increase in liabilities (see Note 7) | (1,450,202) | (3,751) |
| (1,536,580) | (118,440) | |
| Adjustments for items not affecting net cost of operations but affecting appropriations: | ||
| Add (Less): | ||
| Acquisitions of tangible capital assets | 19,542 | 7,894 |
| Net change to accountable advances | 32 | (16) |
| Change in Prepaid Expense | 0 | 0 |
| Suspense accounts (should be zero at year end) | 0 | 0 |
| 19,574 | 7,878 | |
| Current year appropriations used | 2,997,550 | 2,891,980 |
(b) Appropriations provided and used:
| (in thousands of dollars) | Appropriations Provided | |
|---|---|---|
| 2007 | 2006 | |
| Operating expenditures - Vote 1 | 1 805 445 | 1 601 715 |
| Grants and Contributions - Vote 5 | 1 178 285 | 1 247 709 |
| Statutory Amounts | 106 333 | 109 688 |
| Less: | ||
| Appropriation available for future years | (235) | (238) |
| Lapsed appropriations | (92,278) | (66,894) |
| Current year appropriations used | 2,997,550 | 2,891,980 |
(c) Reconciliation of net cash provided by Government to current year appropriations used
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Net cash provided by Government | 2,970,780 | 3,091,284 |
| Revenue not available for spending | 12,597 | 11,234 |
| Change in net position in the Consolidated Revenue Fund | ||
| Refund/reversal of previous year's expenses | 40,390 | 17,224 |
| Justice Canada legal fees | (11,785) | (10,488) |
| Variation in accounts receivable and advance | (6,112) | 27,832 |
| Variation in accounts payable and accrued liabilities | (7,341) | (238,830) |
| Other | (979) | (6,276) |
| 14,173 | (210,538) | |
| Current year appropriations used | 2,997,550 | 2,891,980 |
4. Accounts receivable and advances
Health Canada records receivables from three main sources. As of March 31, amounts due under each of these categories are as follows:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Receivables from External Parties | 21,623 | 21,269 |
| Receivables from Other Government Departments | 13,992 | 8,870 |
| Employee Advances | 106 | 75 |
| Gross receivables | 35,721 | 30,214 |
| Less: Allowance for doubtful accounts on external receivables | (2,249) | (2,854) |
| Net accounts receivable and advances | 33,472 | 27,360 |
5. Tangible capital assets
(in thousands of dollars)
| Capital assets | Opening Balance |
Acquisitions | Disposals/ write-downs/ adjustments |
Closing Balance |
|---|---|---|---|---|
| Land | 1,181 | - | - | 1,181 |
| Buildings | 127,106 | 653 | - | 127,759 |
| Works and infrastructure | 0 | 0 | 0 | 0 |
| Leasehold improvements | 19,277 | - | (4) | 19,273 |
| Machinery and equipment | 106,102 | 13,105 | (469) | 118,738 |
| Computer equipment | 38,434 | 3,241 | (316) | 41,359 |
| Computer software | 4,346 | 464 | 0 | 4,810 |
| Other equipment | 5,431 | 199 | 1 | 5,631 |
| Machinery and equipment | 154,313 | 17,008 | (783) | 170,538 |
| Motor Vehicles | 18,870 | 1,881 | (1,030) | 19,721 |
| Other Vehicles | 1,416 | 0 | 0 | 1,416 |
| Vehicles | 20,286 | 1,881 | (1,030) | 21,137 |
| 322,163 | 19,542 | (1,817) | 339,888 | |
| Accumulated amortization |
Opening Balance |
Current year amortization |
Disposals/ write-downs/ adjustments |
Closing Balance |
| (in thousands of dollars) | ||||
| Buildings | 76,549 | 5,164 | (1) | 81,712 |
| Works and infrastructure | 0 | 0 | 0 | 0 |
| Leasehold improvements | 13,835 | 3,494 | - | 17,329 |
| Machinery and equipment | 73,808 | 6,240 | (467) | 79,581 |
| Computer equipment | 29,206 | 3,502 | (294) | 32,414 |
| Computer software | 3,834 | 295 | 0 | 4,129 |
| Other equipment | 2,673 | 553 | 1 | 3,227 |
| Machinery and equipment | 109,521 | 10,588 | (758) | 119,351 |
| Motor Vehicles | 12,207 | 1,750 | (941) | 13,016 |
| Other Vehicles | 227 | 138 | (1) | 364 |
| Vehicles | 12,434 | 1,888 | (942) | 13,380 |
| 212,339 | 21,134 | (1,701) | 231,772 | |
| Tangible capital assets net book value | Opening Balance |
Closing Balance |
||
| (in thousands of dollars) | ||||
| Land | 1,181 | 1,181 | ||
| Buildings | 50,557 | 46,047 | ||
| Works and infrastructure | 0 | 0 | ||
| Leasehold improvements | 5,442 | 1,944 | ||
| Machinery and equipment | 44,792 | 51,187 | ||
| Vehicles | 7,852 | 7,757 | ||
| 109,824 | 108,116 | |||
| Amortization expense for the year ended March 31, 2007 is $21,134 (2006 - $22,492). | ||||
6. Employee benefits
(a) Pension benefits
The department's employees participate in the Public Service Pension Plan, which is sponsored and administered by the Government of Canada. Pension benefits accrue up to a maximum period of 35 years at a rate of 2 percent per year of pensionable service, times the average of the best five consecutive years of earnings. The benefits are integrated with Canada/Québec Pension Plans benefits and they are indexed to inflation.
Both the employees and the department contribute to the cost of the Plan. The current and previous year expenses, which represent approximately 2.2 times (2.6 in 2005-06) the contributions by employees, amount to:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Expense for the year | 77,728 | 80,743 |
| 77,728 | 80,743 |
The department's responsibility with regard to the Plan is limited to its contributions. Actuarial surpluses or deficiencies are recognized in the financial statements of the Government of Canada, as the Plan's sponsor.
(b) Severance benefits
The department provides severance benefits to its employees based on eligibility, years of service and final salary. These severance benefits are not pre-funded. Benefits will be paid from future appropriations. Information about the severance benefits, measured as at March 31, is as follows:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Accrued benefit obligation, beginning of year | 122,332 | 100,476 |
| Expense for the year | 18,296 | 30,517 |
| Benefits paid during the year | (6,334) | (8,661) |
| Accrued benefit obligation, end of year | 134,294 | 122,332 |
7. Other liabilities
(a) Pension benefits
Other liabilities include allowances and contingencies reflecting $1.023 billion for Hepatitis C litigations and two statutory grants amounting to $430 million as announced in the Budget 2007; (Bill C-52 : $400 million to Canada Health Infoway to support the development of electronic health records and $30 million to Rick Hansen Foundation for the Spinal Cord Injury Transitional Research Network).
8. Contingent liabilities
(a) Contaminated sites
Liabilities are accrued to record the estimated costs related to the management and remediation of contaminated sites where the department is obligated or likely to be obligated to incur such costs. Health Canada has identified sites where such action is possible and for which a liability has been recorded.
| 2007 | 2006 | |
|---|---|---|
| Approximate number of sites for which a liability has been recorded | 5 | 14 |
| (in thousands of dollars) | ||
| Liability recorded for contaminated sites | 3,197 | 3,646 |
Health Canada’s ongoing efforts to assess contaminated sites may result in additional environmental liabilities related to newly identified sites, or changes in the assessments or intended use of existing sites. These liabilities will be accrued in the year in which they become known.
(b) Claims and litigation
In the normal course of its operations, Health Canada becomes involved in various legal actions. Legal proceedings for claims totalling approximately $10,811,022,000 ($10,798,354,000 in 2006) were still pending at March 31, 2007. Some of these potential liabilities may become actual liabilities when one or more future events occur or fail to occur. To the extent that the future event is likely to occur or fail to occur, and a reasonable estimate of the loss can be made, an estimated liability is accrued and an expense recorded on the department’s financial statements.
9. Contractual obligations
The nature of Health Canada's activity results in multi-year contracts and obligations whereby the Department will be committed to make some future payments. Significant contractual obligations that can be reasonably estimated are as follows:
| (in thousands of dollars) | Transfer payments | Non-Insured Health Benefits |
Total |
|---|---|---|---|
| 2007-08 | 126,000 | 22,000 | 148,000 |
| 2008-09 | 97,000 | 31,000 | 128,000 |
| 2009-10 | 96,000 | 20,000 | 116,000 |
| 2010-11 | 58,000 | - | 58,000 |
| 2011-12 and thereafter | 50,000 | - | 50,000 |
| Total | 427,000 | 73,000 | 500,000 |
10. Related party transactions
The department is related as a result of common ownership to all Government of Canada departments, agencies, and Crown corporations. The department enters into transactions with these entities in the normal course of business and on normal trade terms. Also, during the year, the department received services which were obtained without charge from other Government departments as presented in part (a).
(a) Services provided without charge by other government departments:
During the year the department received without charge from other departments, accommodation, legal fees, worker's compensation and the employer's contribution to the health and dental insurance plans. These services without charge have been recognized in the department's Statement of Operations as follows:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Accommodation | 34,914 | 34,481 |
| Employer's contribution to the health and dental insurance plans | 50,980 | 48,176 |
| Worker's compensation costs | 711 | 879 |
| Legal services | 5,339 | 2,039 |
| 91,944 | 85,575 |
The Government has structured some of its administrative activities for efficiency and cost-effectiveness purposes so that one department performs these on behalf of all without charge. The costs of these services, which include payroll and cheque issuance services provided by Public Works and Government Services Canada, are not included as an expense in the department's Statement of Operations.
(b) Payables outstanding at year-end with related parties:
| (in thousands of dollars) | 2007 | 2006 |
|---|---|---|
| Accounts payable to other government departments and agencies | 18,941 | 20,508 |
11. Comparative information
Comparative figures have been reclassified to conform to the current year’s presentation.
12. Subsequent events
On December 14, 2006, the Government and Class Counsel reached a settlement for those Canadians who contracted hepatitis C from the blood system before January 1, 1986 and between July 2, 1990 and September 28, 1998. Under the terms of the agreement, the Government set aside about $1 billion in a special settlement fund which is reflected in the present financial statements. On June 8, 2007, Provincial Superior Courts approved the Settlement and the settlement funds will be transferred to the appointed trustee in the upcoming fiscal year.
Table 13: Responses to Parliamentary Committees, and Audits and Evaluations
| Response to Parliamentary Committee |
Standing Committee on HealthReport #3 “Silicone Gel-filled Breast Implants: Areas of Concern” - tabled September 18, 2006 Committee members heard testimony on the issue of silicone gel-filled breast implants. Witnesses included officials from Health Canada and the Public Health Agency of Canada; former members of Health Canada's Expert Advisory Panel on Breast Implants, Dr. Mitchell Brown and Dr. Paula Chidwick; and Dr. Diana Zuckerman, President of the National Research Center for Women and Families in the United States. Based on the evidence provided by these witnesses, the Committee identified key concerns regarding silicone gel-filled breast implants relating to several areas including safety assessments, special access, informed consent and post-approval surveillance. The Committee acknowledges that these recommendations may require changes to the Medical Devices Regulations and/or other supporting documents. In its response, the Department recognizes the importance of each of the recommendations and the underlying concerns. Health Canada has considered each recommendation carefully and prepared a response to the concerns and advice contained in the Committee's report. The Government's response and actions are in line with the Committee's recommendations. |
| Responses to the Auditor General of Canada, including to the Commissioner of the Environmentand Sustainable Development (CESD) |
|
The Auditor General's (AG's) May 2006 Status Report included Chapter 5 - Management of Programs for First Nations. In this follow-up audit, the AG examined the progress of Health Canada and four other federal organizations in implementing 37 recommendations made between 2000 and 2003 on First Nations issues. The recommendations were included in chapters that covered housing on reserves, economic development, third-party intervention, health care, the food mail program, comprehensive land claims, and reporting requirements for First Nations. The AG also identified factors that appear to have been critical in successfully implementing the recommendations. In its response, the Government agreed that the seven factors identified by the AG were important and that where satisfactory progress has been made on the AG's recommendations, one or more of these factors were present. In addition, the Government noted that these factors constitute an increasingly important part of its approach for the broader Aboriginal agenda. The Government noted that resolving Aboriginal issues remains an extremely difficult challenge, characterized by complex jurisdictional issues. Major reform is complex, requiring phased implementation and the establishment of strong governance and accountability measures in First Nations communities. Nevertheless, the Government will continue to take the critical factors into account when developing approaches aimed at securing a better future for Aboriginal peoples. The AG's November 2006 Annual Report included Chapter 8 - Allocating Funds to Regulatory Programs -Health Canada. The AG examined both the process by which Health Canada decides what resources to allocate to each of its branches, as well as the information used as the basis for resource allocation. In particular, the AG examined how branches allocate resources to three regulatory programs (product safety, drug products and medical devices), and assessed the impact of the Department's resource allocation process on its ability to carry out its regulatory responsibilities in these areas. The audit focused on fiscal years 2003-2004 and 2004-2005. The AG recommended improvements in several areas, including the operational plans for each of the three Health Canada regulatory programs; various aspects of the related resource allocation process, including the establishment of user fees; and the ongoing measurement of and reporting on performance. Health Canada is progressing in the implementation of all the AG recommendations. For example, the Department has strengthened operational planning through the launching of a combined strategic and operational planning process that includes performance measures, indicators and measurable targets, as well as decisions on resource allocations. The completion date for implementing the new process is fiscal year 2007-2008. In addition, Health Canada recently approved a new cost recovery strategy and framework for all its user fees programs, including drugs and medical devices, in order to align with the Treasury Board Policy on Service Standards for External Fees and to develop a full costing model. Work on the cost recovery strategy and framework is expected to be completed by March 31, 2008. Building on stakeholder consultations in 2005, Health Canada is renewing its cost-recovery regime for drugs and medical devices in accordance with the Treasury Board policy and the User Fees Act. Health Canada has launched consultations with stakeholders in 2006-2007 with a goal to complete the work over the next two fiscal years (2007-2008 and 2008-2009). The AG's November 2006 Annual Report also included Chapter 10 - Award and Management of a Health Benefits Contract: Public Works and Government Services Canada and Health Canada. Health Canada is responsible for providing non-insured health benefits such as drugs and medical supplies to eligible First Nations and Inuit people. In 1997, Public Works and Government Services Canada (PWGSC) awarded a contract to First Canadian Health Management Corporation Inc. to provide claim processing services for Health Canada's Non-Insured Health Benefits (NIHB) program. The AG assessed whether PWGSC complied with the government's contracting policy when it awarded the contract and whether Health Canada exercised adequate control over public funds spent on the program. Two of the AG's recommendations were addressed to Health Canada - one related to compliance with sections 33 and 34 of the Financial Administration Act, and the other related to the clarity of the Department's Delegation of Financial Signing Authorities document. As a result of additional audit work conducted between January and September 2006, the AG confirmed that Health Canada had resolved both of these issues. |
| External Audits or Evaluations |
|
Government-wide audits affecting Health Canada conducted by the Auditor General: May 2006 Status Report:
Section III • Supplementary Information Responses to Parliamentary Committees, and Audits and Evaluations (continued) November 2006 Annual Report:
February 2007 Status Report:
Government-wide audits affecting Health Canada conducted by the Commissioner of the Environment and Sustainable Development (CESD) - September 2006 Annual Report:
Audits conducted by the Commissioner of Official Languages (COL):
Audits conducted by the Canada Public Service Agency (CPSA - formerly PSHRMAC):
The audit report is available at this location |
| Implementation of the Federal Accountability Act and Treasury Board's 2006 Internal Audit Policy |
|---|
|
The Federal Accountability Act, which was granted Royal Assent in December 2006, creates, for the first time, a legislative requirement for deputy heads to establish and adequately resource departmental audit functions. This Act provides a legislative basis for the Audit and Accountability Bureau's (AAB) actions to implement Treasury Board's 2006 Internal Audit Policy, which took effect on April 1, 2006. AAB has continued to prepare for the implementation of Treasury Board's 2006 Internal Audit Policy:
|
Table 14: Sustainable Development
| Key goals, objectives, and / or long-term targets of the SDS |
|
Health Canada's 2004-2007 Sustainable Development Strategy is comprised of three themes:
Under each theme are various objectives and targets. During 2006-2007 progress was made in all three areas. |
| How key goals, objectives, and / or long-term targets help us achieve our strategic outcomes |
|
Health Canada has four Strategic Outcomes as set out in the 2007-08 RPP:
Targets established under theme one directly support all four Strategic Outcomes. Targets under theme two also support the full range of outcomes, although primarily by strengthening the knowledge base and, in turn, management and decision making practices. Theme three supports the greening of HC operations and activities. |
| Targets and Progress |
|
Health Canada has been successful in meeting most of the commitments it set out to achieve in SDS III, and has identified activities that were integrated into SDS IV to support the completion of the remainder of SDS III targets. For detailed progress information on each specific target of SDS III, see Health Canada's Sustainable Development Strategy 2004-2007: Becoming the Change We Wish to See Final Report on Accomplishments 2004-2007. To obtain a copy of this report please contact the Office of Sustainable Development, Health Canada, at osd@hc-sc.gc.ca or (613)954-3859. Theme 1 Accomplishments The objectives and targets under this theme focussed on accelerating the creation of social and physical conditions that maintain and enhance population health. Some of our key accomplishments in 2006-2007 to address these priorities included: Health Canada successfully met its targets related to drinking water quality in Canada through development of Drinking Water Guidelines (target 1.1.1) in partnership with federal, provincial and territorial departments of health and environment, the development of a national protocol for addressing water-borne contamination and illness events, revising the Procedure Manual for Safe Drinking Water in First Nations Communities South of 60 and developing a risk assessment tool for drinking water in First Nations communities. Health Canada worked with other federal departments to determine the vulnerability of Canadians to climate change impacts through selected case studies, methods development and literature review (target 1.1.4). A synthesis report has been developed and the final version is planned for release in November 2007. Health Canada developed and updated science-based guidelines and standards to improve the safety of the food supply and reduce food-borne illness (target 1.1.5), The Department also began implementation of the Aboriginal Health Transition Fund to devise new ways to improve, integrate and adapt health services to better meet the needs of all Aboriginal peoples (target 1.2.2). Significant progress was realized by the Pest Management Regulatory Agency (PMRA) in re-evaluating registered products to ensure they meet current safety standards. Proposed regulatory decisions were published for 14 pesticide active ingredients, while regulatory decisions were finalized and published for 17 pesticide active ingredients. In collaboration with Agriculture and Agri-Food Canada, PMRA constructed a website to report on risk reduction projects for priority crops (target 1.1.7). Theme 2 Accomplishments The objectives and targets under Theme 2 were designed to broaden Health Canada employees' knowledge of sustainable development issues and provide practical tools for integrating SD into all decision-making, programs, and policies. Work continues on integrating SD into the planning processes at the Departmental, Branch, Regional and Agency levels, to ensure that SD is not considered an “add-on” to overall operations. Efforts in SDS III 2004-2007 focused on development and delivery of Sustainable Development (SD) training and communication tools to new and existing HC employees in order to raise awareness and improve integration of SD (targets 2.1.1 & 2.1.2). Over 200 staff were trained in Strategic Environmental Assessment (SEA) preparation and their responsibilities under the Cabinet Directive on SEAs. Health Canada completed a detailed SEA on the Government's Chemicals Management Plan. Health Canada has drafted an SD Policy Lens, which will undergo a pilot test in 2007, with the aim of improving SD considerations embedded in policies, plans and programs. Theme 3 Accomplishments The objectives under this theme strengthened Health Canada's commitment to decrease adverse environmental impacts resulting from the Department's operations and to promote our social responsibility in communities with Health Canada facilities. Targets focused on greening of government operations and included providing more information to assist managers and employees when they conduct their daily activities and operations, through the production of two departmental guidebooks: Making a Difference in Our Facilities and Our Environment: A Guide on Environmental Management Best Practices for Health Canada and Agency Facilities; and Making a Difference: A Departmental Guidebook on Pollution Prevention for Health Canada and Agency Employees (targets 3.1.1 & 3.2.1). Health Canada continued to follow up, implement and report on recommendations outlined in its guidebook to improve the management of environmental impacts at its laboratories and health facilities as part of its Environmental Management System (target 3.1.3). Best practices and initiatives on SD were implemented in the regions including practising zero-waste catering, supporting fair trade products, using photocopy and printer paper with at least 30 percent post-consumer content, and green procurement (target 3.2.3). |
| Adjustments |
|
In April 2006 the federal government launched a new policy on Green Procurement. This policy encourages the selection of goods and services that are the least likely to have a negative impact on the environment during their entire 'life cycle' (production, usage and disposal). Health Canada has begun implementing this policy across the department by collecting baseline
information, communicating the policy and tools available to assist employees to implement the policy and by including specific Green Procurement targets into SDS IV. The Department will continue to report progress annually toward implementing this policy.
Health Canada considers the SDS to be a living document that evolves over time in response to emerging opportunities and to formal recommendations and audits. Although considerable progress was made in reaching the objectives and targets in SDS III, two targets (1.1.5 and 2.1.5) were carried over to the next strategy, SDS IV, as they were not fully met within the 2004-2007 period. SDS III had a stronger performance management framework than previous strategies. A generic example results chain was developed to outline the logic and expected outcomes for SDS III, and measure performance against indicators. However, while some targets fit well within the results chain, others did not. To address performance measurement issues in SDS IV, a concerted effort was placed on ensuring targets are “SMART”: Supportive of strategic themes and specific; Measureable; Action-oriented and achievable; Resourced (i.e. human and fiscal allocations) and relevant; Time-bound by deadlines and/or criteria. This will ensure a simple and accountable system of measuring progress. Each objective and target is associated with a clear and measurable indicator which will provide a reliable estimate and whether the target has been reached. |
Table 15 and 16
Detailed information on Procurement and Contracting (Table 15) and Service Improvements (Table 16)
Table 17: Horizontal Initiatives
| Name of Horizonatal Initiative |
|
Table 18: Travel Policies
| Comparison to the TBS Special Travel Authorities |
| Health Canada follows and uses the TBS Special Travel Authority Parameters. |
| Comparison to the TBS Travel Directive Rates and Allowances |
| Health Canada follows and uses the TBS Travel Directive Rates and Allowances. |
Table 19: Storage Tanks
Supplementary information on storage tanks can be found here.
Section IV: Other Items of Interest
Health Canada's Regional Structure and Operations
In January 2006, Health Canada created a new Public Affairs, Consultation and Regions Branch. The creation of the Branch afforded the Department the opportunity to better bring national and regional perspectives to policy and program development, service delivery strategies, and communication and consultation functions. It allowed the Department to clearly define and clarify roles, responsibilities and accountabilities in a manner that contributes to the overall success of the Department and allows for a greater degree of coherence and integration of all operations in the regions.
Health Canada's seven regional operations (British Columbia, Alberta, Manitoba/Saskatchewan, Ontario, Quebec, Atlantic, and Northern) represent the face of the Department to Canadians through their role as front- line service and information providers, as guardians and regulators and by the delivery of health services and programs in First Nations and Inuit communities. These roles allow Health Canada to maximize the reach and effectiveness of departmental programs and services as well as to respond to the varied needs of diverse communities that the Department serves across the country. The creation of a new single Northern Region recognized the distinct program and service delivery challenges and opportunities of people in the territories.
Regions achieved an ambitious agenda of outreach and engagement with partners and stakeholders including provincial and territorial government departments, regional health authorities, health boards, research and academic institutions, non-governmental organizations and First Nations and Inuit governing bodies. This commitment was illustrated by the negotiations that led to the signing of the First Nations Tripartite Health Plan between the British Columbia Government, Health Canada and the First Nations Leadership Council. This 10 year plan will ensure more coordinated services among the three partners and related governance development to support stronger First Nations leadership in health.
Through well-established networks, Health Canada's regions continued to ensure that the Department was informed of local issues and concerns so that policy and program development considered and reflected the expectations, needs and issues of Canadians in all parts of the country.
Health Canada Communications and Outreach to Canadians
Health Canada communications focused on providing timely, reliable, relevant and culturally appropriate health information to Canadians. More than a million Canadians visited the website monthly for information. Public opinion research was gathered to support development of programs, policies and regulations, and targeted social marketing campaigns encouraged healthy behaviours.
Supporting Health Canada's Programs and Services
The 2006-2007 Report on Plans and Priorities (RPP) included commitments to corporate services and management practices.
Human Resources
Health Canada has been acknowledged for its good governance and training structure around Public Service Modernization Act implementation, the Department continued to provide training and improve policies and tools to support the new approaches set out under the Act. Branches identify human resource priorities and strategies based on business objectives, and supported by detailed demographic and environmental scanning information.
Overall, Health Canada received ratings of 'Acceptable' on the work force and workplace elements of the 'People' components of the Management Accountability Framework (MAF) which were largely based on 2005 employee survey results.
Information Technology
As set out in the RPP, The Way Forward initiatives resulted in many changes to IT management in Health Canada. This two-year departmental initiative adopted national standards and integrated, consolidated and rationalized IT resources. Projects included consolidating and reducing the number of servers and reducing IT computing facilities from 50 to four. The majority of projects were completed, with the remainder slated for completion in 2007-2008. We also were engaged in the government-wide IT Shared Services Initiative to improve delivery of internal administrative services, increase operational efficiency and consider transaction-based services that could be delivered by a common service provider. We have already transferred responsibility for some services to Public Works and Government Services Canada (PWGSC).
Promoting Management Accountability and Operational Planning
Health Canada continued strengthening financial management, accountability and control, as well as resource allocation. Under our Financial Management and Control Framework (FMCF) project, we introduced a Budget Management Framework. We also launched the Readiness Assessment and Certification Initiative as part of the Financial Management Renewal Initiative led by the Office of the Comptroller General, which supports achievement of Federal Accountability Act goals. Phase 1 of the Department’s automated Contract Requisition and Reporting System was implemented and is aligned with the government's priority of improving accountability.
Improvements to operational planning were a major element supporting clearer management accountability. Introduced as a pilot in June 2006, the Departmental Operational Planning (DOP) is a FMCF priority. We are moving to a standardized planning framework across the Department that will clearly demonstrate linkages between priorities, planned activities, expected results and proposed resource allocation. These plans highlight risks and include mitigation strategies needed to reflect challenges facing departmental operations
These actions were rooted in departmental analyses of areas for improvement in financial management practices and were reinforced as a result of the recommendations in Chapter 8 of the 2006 Report of the Auditor General -Allocation of Resources to Regulatory Programs as well as the 2006 MAF Assessment. In that assessment, Treasury Board Secretariat (TBS) recognized us fo improved management in information technology, citizen-focused services, effective procurement and extra-organizational contributions. TBS commended Health Canada for our role in supporting TBS' priority of streamlining the Government’s Policy Suite.
Risk Management
Health Canada undertook exercises relating to risk management such as the annual combined update of our Corporate Risk Profile and Internal Environmental Scan, the update of the Departmental Multi-Year Risk-Based Audit Plan 2006-2007 to 2008-2009 and the testing of the Departmental Business Continuity Plan in the Event of a Pandemic Influenza Outbreak. Health Canada and the Public Health Agency of Canada’s Strategic Risk Communications Framework established an in-depth training plan to provide in-depth risk communications training. Extensive risk management processes supported The Way Forward and human resource activities described elsewhere in this section.
Privacy
We processed 2,200 requests under the Access to Information Act and the Privacy Act making Health Canada one of the top three departments in terms of requests received and processed. We improved our efficiency in responding to these requests, meeting deadlines in 86 percent of cases by the end of 2006-2007, up from 78 the year before. We were on track to achieve an "ideal compliance" status of 95 by fall 2007.
We increased awareness of Access to Information and Privacy principles by providing a training program that reached 500 Health Canada and Public Health Agency of Canada employees. We also oversaw a Privacy Impact Assessment process, initiating seven Privacy Impact Assessments and three Preliminary Privacy Impact Assessments. These are part of our efforts to ensure that personal information entrusted to the Department is protected. Our newly revised Privacy Impact Assessment Tool Kit was cited by the Office of the Privacy Commissioner as an excellent guide for procedures and practices.
The Office of the Privacy Commissioner the training programs will go a long way in ensuring that the Department “becomes a leader in protecting the privacy of Canadians in the delivery of critical health services. Indeed the training program is one of the more comprehensive suites currently available within the federal government, and could very well become a benchmark for future employee privacy training.”
Values and Ethics
The departmental emphasis on values and ethics included raising awareness among employees of ethical issues and engaging them in dialogue about those issues. Other efforts assisted staff in addressing specific concerns and resolving conflicts. More than 2,500 employees participated in activities to educate, promote or foster ethics and informal conflict management in the workplace. In addition, over 400 employees used Internal Ombudsman services which helped to build a positive, open and transparent working environment.
The Health Canada Sustainable Development Strategy
Sustainable development is implicit in Health Canada's plans and priorities. Health Canada's Sustainable Development Strategy III (SDS III 2004-2007): Becoming the Change We Wish to See is described in detail in Table 14.
We also carried out planning to create SDS IV, which will build on lessons learned as well as new directions in government-wide efforts. Sustainable development training and implementing policy and planning tools will raise awareness and improve integration of social, economic and environmental considerations in the work the Department performs.
Advancing the Science Agenda
The Office of the Chief Scientist (OCS) was created in 2001 to strengthen our ability to perform and use science. Led by the Chief Scientist, the OCS provides leadership for and promotes awareness of Health Canada's science and research and encourages and supports the science community within and outside Health Canada. This helps to ensure that the Department has the scientific information needed to make health-related decisions. The OCS undertakes activities in three key areas:
Science advice - Promoting the effective use of science in policy making: assisting the Department in employing quality science advice in its policy and regulatory decisions;
Science management - Enhancing science capacity and quality: Encouraging due diligence and ensuring Health Canada has the science capacity it needs to meet current and emerging challenges; and
Science promotion - Raising awareness and understanding of science conducted at Health Canada: Improving stakeholder and public understanding of departmental science and its contribution to the health and safety of Canadians.
Science Advice
The OCS is responsible for the provision of science advice to senior departmental officials. It continued its role as secretariat to the Science Advisory Board (SAB). The SAB provides the Minister of Health with independent, expert advice on the science performed and used by Health Canada. This advice was considered in developmentof a departmental science and technology (S&T) strategy, integrated approaches to health and the environment, pharmacosurveillance and the implications of a federal S&T Strategy on the Health Portfolio.
As a leading science-based department, Health Canada must ensure that its research is conducted in a responsible manner. Health Canada's Research Ethics Board (REB), an independent body of experts, ensures that departmental research involving humans meets the highest ethical standards. OCS also provides secretariat support to the REB, which reviewed 132 research protocols in 2006-2007. Approximately 45 percent of these protocols came from the Public Health Agency of Canada and the others were submitted by Health Canada researchers.
The OCS worked with stakeholders in the research community to establish the Canadian Research Integrity Committee (CRIC). The OCS supported the CRIC in hosting a national workshop in January 2007 to discuss research integrity and the potential for a national approach.
Science Management
Sound science management requires good sharing of information and coordination of efforts between Health Canada branches. A key mechanism is the Departmental Executive Committee's Subcommittee on Science, which the Chief Scientist chairs and has ADM-level membership from all branches.
The OCS initiated planning for the S&T Strategy, referred to above, which will be developed in 2007-2008. The Strategy will seek to strengthen management of science issues across the department and in alignment with government-wide science directions.
Fostering strategic partnerships and linkages with external partners/stakeholders is critical to accessing the science and augmenting the science capacity our Department needs to implement its mandate. The OCS worked with PHAC and Canadian Institute of Health Research (CIHR) to identify opportunities for increased collaboration and information sharing. The OCS also supported development of an integrated list of health services and policy research priorities. In addition, the OCS provided advice and guidelines for departmental researchers and scientists on collaborative arrangements with external stakeholders.
With OCS coordination, Health Canada participated in development of the new federal S&T Strategy. Other interdepartmental initiatives dealt with recruitment and development of scientific personnel. These horizontal science initiatives improve understanding within the federal community of the importance of the regulatory science that is central to Health Canada efforts to maintain and improve the health of Canadians.
The OCS coordinated Health Canada's activities with respect to the OECD Principles of Good Laboratory Practice (GLP), which promote test data of comparable quality to enable mutual acceptance of data for regulatory purposes among different countries. To complement the existing GLP programs for pesticides and industrial chemicals and comply with Canada's OECD obligations, the OCS oversaw GLP implementation approaches for health products and food additives.
In order to strengthen the Department’s research capacity, six additional postdoctoral fellowships were offered and managed by OCS. In October 2006, OCS assumed management of the NSERC Visiting Fellowship Program for the Department with 24 Fellows. These programs bring new ideas and cutting-edge science into the Department in the person of the Fellows, who gain insight into the needs and operations of Health Canada. These programs also identify potential employees.
The Intellectual Property and Technology Transfer Office assisted 25 scientists with intellectual property issues. A one-day workshop on Intellectual Property was held for departmental scientists and managers. A web-based system called “Flintbox” to market patented inventions was implemented. An award for departmental inventors was also initiated.
Science Promotion
The OCS organized the annual Health Canada Science Forum held in Ottawa in October 2006 around the theme: “Keeping our "I"s on the Future: Innovation, Integration, Information and International”. This event helped raise awareness of the excellent research performed in the department and its contribution to policy and regulatory decision making. The Forum also facilitated linkages and information sharing between Health Canada researchers and decision makers and counterparts from across Canada.
Section V: Other Information
Departmental Contact Information
Northern Region
60 Queen St., Suite 1400
Ottawa, Ontario
K1A 0K9
General Inquiries: 1-866-509-1769
Non-Insured Health Benefits Inquiries: 1-888-332-9222
Fax: (613) 954-9953 or 1-800-949-2718
Atlantic Region
1505 Barrington Street, Suite 1917
Halifax, Nova Scotia
B3J 3Y6
Telephone: (902) 426-2038
Facsimile: (902) 426-3768
Manitoba and Saskatchewan Region
Suite 450 - 391 York Avenue
Postal Locator B200
Winnipeg, Manitoba
R3C 4W1
Phone: (204) 983-2508
Facsimile: (204) 983-3972
Quebec Region
Complexe Guy Favreau
East Tower, Suite 200
200 René Lévesque Blvd. West
Montreal, Quebec
H2Z 1X4
Telephone: (514) 283-5186
Facsimile: (514) 283-1364
Alberta Region
Canada Place, Suite 730
9700 Jasper Avenue
Edmonton, Alberta
T5J 4C3
Telephone: (780) 495-6815
Facsimile: (780) 495-5551
Ontario Region
180 Queen Street West,
Toronto, Ontario
M5V 3L7
Telephone: (416) 973-4389
Toll free: 1-866-999-7612
Fax: (416) 973-1423
British Columbia and Yukon Region
757 West Hastings Street, Room 235
Vancouver, British Columbia
V6C 1A1
Telephone: (604) 666-2083
Facsimile: (604) 666-2258
National Capital Region
Health Canada 0900C2
Podium Level Brooke Claxton Building
Columbine Drive
Ottawa, Ontario
K1A 0K9
Telephone: (613) 957-2991
Facsimile: (613) 941-5366
Table 7B: Policy on Service Standards for External Fees
Healthy Environments and Consumer Safety Branch (HECS)
|
External Fee |
Service Standard |
Performance Result |
Stakeholder Consultation |
|
National Dosimetry Services Product, Services and Fee Structure (NDS P, S&F) |
Provide timely, responsive and reliable customer services to 95,000 workers in 13,000 groups: Registration and inspections of incoming dosimeters within 48 hours Exposures over regulatory limits reported within 24 hours Dosimeters leave NDS premises 10-13 working days prior to exchange date Message call backs (phone, e-mail) within 24 hours Updated account information within 48 hours Additional request dosimeters shipped within 24 hours |
Provided timely, responsive and reliable customer services to 95,000 workers in 13,000 groups. The standards were met as follows: |
NDS manages client contacts ( 55, 000 this year) on a daily basis through the Call Centre system. This allows NDS to measure level of service satisfaction as well as insight into new requirements for products and services. NDS documents and assess customer feedback (compliments and criticism) using a centralized electronic database directly connected to the Call Centre system. Additional information on service is obtained during regular contact sessions with the client and, as required, through exit questionnaires. This year, NDS will be engaging selected clients in service specific questionnaire. On a basis of over 500,000 dosimeter readings annually, NDS satisfaction rate is more than 99.9%. |
|
Deratting Services |
Health Canada provides 7-day service in designated ports and all requests are responded to within 48 hours. |
100% of requests received were responded to within 48 hours or less. |
There were no changes to the service standards in 2006-2007. The International Health Regulations (WHO) requires that the new Ship Sanitation Certificates be implemented by June 15, 2007. The shipping industry was consulted by HC in the 2006-07 year. Fees will not change until after December 15, 2007 when a new costing analysis will be completed. |
|
Cruise Ship Inspection Program |
Periodic inspections done a minimum of once a sailing season on ships in Canadian waters. |
See Notes 2 and 3 below. |
There were no changes to the service standards in 2006-2007. Health Canada meets with stakeholders on an annual basis to review and discuss any proposed changes to the service standards. The standards are consistent with the CDC/VSP (Vessel Sanitation Program) administrative guideline and criteria for inspections, and any changes would be synchronized to harmonize
the process with the U.S. |
|
Common Carrier Inspection (e.g. trains, ferries, airports/airlines, seaports) |
See Note 3 below. |
See Note 4 below. |
Service standards are negotiated and included in MOUs/contracts; service standards/MOUs remain unchanged in 2006-07. Stakeholders consulted at the annual HC-industry meeting. |
|
Employee Assistance Services (EAS) |
As per formal agreement, varies depending on customer organization's requirements, needs and EAS capacity to meet these. |
EAS is an Accredited service. Voluntary satisfaction surveys, customer surveys and follow-ups with clients and customers are done on a regular basis. Results are shared at year end with report to each customer, as per formal agreement. |
Customer survey and meeting with customer at least once a year. Formal agreement to renew contractual or MOU-type agreement done annually or every two years. Utilization data given at least annually to each customer. |
|
Medical Marihuana Cannabis seeds |
Dried marihuana Cannabis seeds |
Dried marihuana |
As a result of comments received from the first users of marihuana produced under contract for Health Canada, a "bud" only product with increased potency has been distributed by Health Canada since May 2004. Additional changes to the physical attributes of dried marihuana resulting in a marihuana product with higher humidity and larger particle size were made
September 2005, thus increasing the quality of the product. |
Note 1: In total, 675 Derat certificates were issued in 2006-2007. See table below for details on service standards.
| Day of the Week |
Prior Notification Required |
|
Weekday Service - Designated Ports |
24 hours |
|
Weekend Service - Designated Ports |
48 hours |
|
Regular Weekend Service - Designated Ports |
For service on Saturday, notice must be received Thursday by 1300 hours local time. |
|
Holiday Weekend Service - Designated Ports |
When Friday is the statutory holiday
When Monday is the statutory holiday
|
|
Prior Notice for Service - Non-designated Ports |
72 hours prior notice is requested for service at non-designated ports. |
NOTE: The fee for short notice service i.e. less than 24 hours for week days, less than 48 hours for weekends, at both designated and non-designated ports, will be the normal fee plus a 25% surcharge.
Note 2: Health Canada publishes scores obtained from the Cruise Ship Inspection Program at: http://www.hc-sc.gc.ca/hl-vs/travel-voyage/general/inspection/2006-cruise_ship_inspection-navires_croissieres_inspection_e.html
Note 3: In regards to service standards, Cruise Ship and Common Carrier Inspections are performed following procedures and protocols that have been published and distributed to clients. Health Canada's protocols are consistent with programs in other countries. Copies of the inspection protocols for these programs may be requested by e-mail at: phb_bsp@hc-sc.gc.ca.
Note 4: Service Standards for Conveyance Inspection Program
| Conveyance Inspection Program |
Service Standard |
Performance Result |
|
Passenger Train - On Board |
Periodic inspection done on each passenger train line as determined by MOU between Health Canada and passenger train industry. |
100% of reports provided within 10 working days. |
|
Passenger Train - Off Board |
Sanitation inspection done twice a year. |
100% of reports provided within 10 working days. |
|
Flight Kitchen |
Scheduled number of announced audits per year is based on the number of meals prepared by the kitchen. |
100% of reports provided within 10 working days. |
|
Ferry - On Board Food |
Unannounced inspections as per predetermined contractual obligations. |
100% of reports provided within 10 working days. |
|
Ferry - Potable Water |
Unannounced inspections as per predetermined contractual obligations. |
100% of reports provided within 10 working days. |
B. Other Information
National Dosimetry Services
NDS billed for $ 5.2 M in 2006/2007, and collected $ 4.5 M in net vote/re-spendable cash. A plan is being developed to integrate a new product, - the next generation dosimeter - by 2008/9., as well as modifications to address gaps in financial performance ( ie, cost of living), business capacity ( ie, competition), and client demands expectations for enhanced levels
of other products and services.
As established pursuant to the Policy on Service Standards for External Fees:
- Service standards may not have received Parliamentary review; and
- Service standards may not respect all performance standard establishment requirements under the UFA (e.g. international comparison; independent complaint address)
- Performance results are not legally bound to section 5.1 of the UFA regarding fee reductions for underachieved performance.
Health Products and Foods Branch
NOTE: This table will not be included in the printed DPR
|
A. External Fee |
Service Standard |
Performance Result |
Stakeholder Consultation |
|
Authority to Sell Drugs Fees |
120 calendar days to update the Drug Product Database following notification |
96% within 120 calendar days |
The Health Products and Foods Branch (HPFB) is actively engaging stakeholders in the development of a cost recovery framework, including relevant service standards. An initial framework of cost recovery fees and service standards was developed in 2006-2007 and presented for consultation in April 2007. Ongoing consultations are scheduled for 2007-2008, with fees and service standards targeted for implementation in 2008-2009. |
|
Certificates of Pharmaceutical Product (Drug Export) Fees |
5 working days to issue certificate |
95% certificates issued within 5 working days |
|
|
Drug Establishment Licensing Fees |
250 calendar days to issue / renew licence |
90% licences issued/renewed within 300 calendar days |
|
|
Drug Master File Fees |
30 calendar days |
100% within 30 calendar days |
|
|
Drug Submission Evaluation Fees (Pharmaceuticals & Biologic Products) |
NDS: Priority NAS = 180 |
Average review time to first decision (calendar days) Pharmaceutical Products Biologic Products |
|
|
Medical Device Licence Application Fees |
Time to first decision (calendar days) |
Time to first decision (calendar days) |
|
|
Fees for Right to Sell a Licensed Medical Device |
20 calendar days from deadline for receipt of annual notification to update the Medical Devices Active License Listing (MDALL) database |
100% within 20 calendar days |
|
|
Medical Device Establishment Licensing Fees |
120 calendar days to issue / renew licence |
90% licences issued/renewed within 120 calendar days |
|
|
Veterinary Drug Evaluation Fees |
Review time to first decision (calendar days) NDS, ABNDS = 300 |
Average review time to first decision (calendar days) |
Fees and service standards related to veterinary drug product activities are under development, but no specific proposals have been presented to stakeholders. |
|
B. Other Information: |
|||
Pest Management Regulatory Agency
In order to reduce the volume of printed material, this table is not to be included in the printed DPR.
|
A. External Fee |
Service Standard |
Performance Result |
Stakeholder Consultation |
|
Fees to be paid for Pest Control Product Application Examination Service |
Target is 90% of submissions in all categories to be processed within time shown. |
Category A = 94% |
Stakeholder consultation conducted annually when required. |
|
Fees to be paid for the right or privilege to manufacture or sell a pest control product in Canada and for establishing a Maximum Residue Limit in relation to a pest control product.
|
100% of all fees for the right or privilege to manufacture or sell a pest control product in Canada have been invoiced by April 30th of the fiscal year |
N/A |
All stakeholders have been consulted on the proposed service standard for invoicing clients |
|
B. Other Information |
|||
Corporate Services Branch (CSB)
|
A. External Fee |
Service Standard |
Performance Result |
Stakeholder Consultation |
|
Fees charged for the processing of access requests filed under the Access to Information Act (ATIA) |
Response provided within 30 days following receipt of request; response time may be extended pursuant to section 9 of the ATIA. Notice of extension to be sent within 30 days of receipt of request. |
Of 2.017 requests, 1,643 (81.5%) were completed during 2006-2007. The Department was able to respond within 30 days or less in 626 (38.1%) of completed cases. Response times for the remaining cases were 280 (17.0%) within |
N/A |
|
B. Other Information |
|||
Table 8: Progress Towards Results on Major Regulatory Initiatives
|
Regulations |
Expected Results |
Performance Measurement Criteria |
Results Achieved |
|
Health Products and Food Branch |
|||
|
Food and Drug Regulations |
Facilitation of greater consumer choice and industry innovation by revision of regulations on the addition of vitamins and mineral nutrients to foods taking into account the role of nutrient addition to foods, consumer needs and expectations, and industry requests. |
Improved nutritional quality of the Canadian food supply. Availability of increased number of choices of foods with added vitamins and mineral nutrients. |
Proposed regulatory amendments are finalized. Publication of the proposed amendments is anticipated for Fall 2007 for public consultation (pre-publication in the Canada Gazette, Part I). |
|
Food and Drug Regulations |
Enhanced protection of allergic consumers through mandatory labelling of specific food allergens, gluten sources and sulphites when present at 10 parts per million or more, on the labels of prepackaged food products, whether they have been added directly or indirectly. |
Reduced number of adverse reactions to prepared and prepackaged foods containing specific allergens, gluten sources and sulphites. Increased consumer awareness of the presence of specific allergens, gluten sources and sulphites in prepared and prepackaged foods. |
Proposed regulatory amendments are being developed to implement the new policy. Publication of the proposed amendments is anticipated for Fall 2007 for public consultation (pre-publication in the Canada Gazette, Part I). |
|
Food and Drug Regulations |
Providing safe handling information on the labels of meat products which, due to their raw state, can introduce disease-causing bacteria directly to consumers as well as the food preparation environment. |
Reduction of food borne illness as a result of providing safe handling information on the labels of these products. |
Proposed regulatory amendments are being developed to implement the new policy. |
|
Food and Drug Regulations |
Addition of two diet-related health claims regarding; 1-fruits, vegetables and whole grains and reduced risk of heart disease and 2- folic acid and reduced risk of neural tube defects to list of claims that manufacturers can use to promote healthy foods. |
Healthier eating practices would be monitored through the use of dietary surveys. The number of foods carrying the approved health claims. |
Health Canada posted on its website, the “Position Paper on Five US Health Claims Considered for Use in Canada” during Fall 2006 which details its plan to allow the two new health claims. Once the policy development is completed, the regulatory amendments will be initiated. |
|
Food and Drug Regulations |
Saccharin permitted as a food additive in a limited number of foods. |
Availability of an additional intense sweetener to allow a wider range of dietetic food products for the benefit of consumers who wish to consume these products. |
Health Canada completed its safety evaluation. Planning for preparation of regulatory amendments. |
|
Food and Drug Regulations |
Additional label information on levels of caffeine in prepackaged beverages, including energy drinks containing caffeine, to allow consumers to make an informed choice about their caffeine intake. |
Increased consumer awareness of the levels of caffeine in beverages sold in Canada. |
Health Canada is considering policy options in regard to requirements of additional label information to consumers. |
|
Food and Drug Regulations |
Providing additional labelling information regarding consumption of unpasteurized juice products. |
Reduction of food borne illness related to the consumption of prepackaged unpasteurized juice products. Increased consumer awareness of the potential microbiological hazards associated with consumption of unpasteurized juice. |
Health Canada conducted public consultation regarding labelling statements and continued its policy development. Preparation of regulatory amendments will be initiated. |
|
Food and Drug Regulations |
Revision and updating of the safety and labelling requirements for prepackaged water and ice products. |
Industry compliance with the revised regulations. |
Proposed regulatory amendments are being developed for public consultation (publication in the Canada Gazette, Part I). |
|
Food and Drug Regulations (Prohibition of Importation for Personal Use) |
Further restrict the importation of veterinary drugs to include the personal importation of drugs intended to be used in food-producing animals to avoid potentially harmful residues in food products from animals treated with these drugs. |
Reduced incidence of harmful drug residues being detected in animals treated with veterinary drugs imported under personal use circumstances. |
The Task Force on Personal Use Importation of Veterinary Drugs has been formed to gain the best possible advice from affected stakeholder groups. The Task Force will provide a report to Health Canada outlining concrete recommendations and strategies to address the issues surrounding the personal importation of veterinary drugs, by the end of August 2007. |
|
Food and Drug Regulations (Carbadox) |
Further restrict the sale of products containing Carbadox for sale in Canada to avoid potentially harmful residues in food products from animals treated with this drug. |
Reduced availability of food products containing Carbadox residues or its metabolites derived from food-producing animals. |
The Canadian Food Inspection Agency enhanced monitoring program for Carbadox will be in place July 2007. Health Canada is to reassess the need for regulatory action (in a year). |
|
Food and Drug Regulations |
The proposed regulatory provisions will provide Health Canada with a legal mechanism to authorize access to unauthorized drugs for mass distribution (block release) for either an immediate emergency or in anticipation of a health emergency. |
Health Canada (HC) will have a greater capacity to plan and prepare for public health emergencies such as infectious diseases and emerging chemical, biological, radiological and nuclear (CBRN) threats. Efforts in emergency preparedness activities will ensure a stockpile of drugs be made readily available with proper regulatory authority. This amendment will provide Health Canada with regulatory authority to allow access to unauthorized drugs in anticipation of a health emergency. |
Proposed regulatory amendments are being developed for public consultation (publication in the Canada Gazette, Part I). |
|
Medical Device Regulations (Introduce additional requirements for investigational testing for medical devices) |
Minimize risks to participants in medical device investigational testing research, which may lead to health benefits for Canadians. |
Decreased risk to participants enrolled in medical device investigational testing research. |
Discussion paper developed to obtain input from stakeholders on the issues, analysis and recommended option. |
|
Medical Device Regulations |
Federal Government is working with provinces and territories to develop approaches in order to mitigate the risks associated with the reprocessing of single-use devices. |
Performance measurement criteria under consideration by the FPT working group.
|
|
|
Food and Drug Regulations |
Allows industry to label and advertise Schedule A preventative claims approved by Health Canada for non-prescription drugs and Natural Health Products (NHPs). Modernization of Schedule A list of disorders and diseases. |
Number of approved preventative claims being labelled for non-prescription drugs and NHPs. |
|
|
Food and Drug Regulations (Regulations amending the data protection provisions) |
Amend the data protection provisions of the Food and Drug Regulations to provide effective data protection for eight years for innovator drugs that contain medicinal ingredients not previously approved for sale in Canada. An additional six months will be provided for submissions that include paediatric studies. |
Provide a minimum predictable automatic protection for innovative new drugs so that these products are available to Canadians. Encourage submission of paediatric information. The number of drugs listed on register of innovative drugs increases. |
Final regulations registered (publication in the Canada Gazette, Part II) in October 2006. Twenty seven drugs listed on register of innovative drugs, 2 of which will have the paediatric extension. |
|
Food and Drug Regulations |
Reflect current methods and practices used to collect human plasma as well as the list of transmissible diseases for which tests must be performed in order to maximize the safety of plasma and plasma donors. |
Regulations consistent with current methods and practices used to collect human plasma. |
Regulations were finalized using public consultation results (pre-publication in the Canada Gazette, Part I) and were registered (published in the Canada Gazette, Part II) in December 2006. |
|
Food and Drug Regulations |
The new CTO Regulations address safety in the processing and handling of these products, resulting in improved protection of the health and safety of Canadian transplant recipients, while at the same time helping to ensure the availability of these life saving and life enhancing products. |
Improved protection of the health and safety of Canadian transplant recipients. |
Regulations were finalized using public consultation results (pre-publication in the Canada Gazette, Part I) to be registered (published in the Canada Gazette, Part II) in June 2007. Regulations will come into force on December 7, 2007. |
|
Food and Drug Regulations |
The amended regulations will eliminate regulatory burden for the performance of certain limited basic clinical research studies, while helping to ensure that patient safety is not compromised. |
More appropriate regulatory requirements placed on researchers conducting basic clinical research with positron emitting radio-pharmaceuticals in Canada. |
Proposed regulatory amendments are being developed for public consultation (pre-publication in the Canada Gazette, Part I) targeted for Fall 2007. |
|
Food and Drugs Act
|
Balance the need for safe blood and blood components with the need to ensure the availability of blood and blood components for transfusion. The new regulations will include basic safety requirements, adverse event reporting requirements and a compliance and enforcement strategy. |
Greater harmonization achieved in Canada related to collection, handling and post-market surveillance of blood and blood components. Improvement in the protection of the health and safety of Canadian blood and blood component donors and recipients. Requirements updated on a timely basis as new technologies/ products/issues emerge. |
Proposed regulatory amendments are being developed for public consultation (pre-publication in the Canada Gazette, Part I) targeted for first half of 2008. |
|
Food and Drugs Act |
Formal mechanism to approve products which only contain substantial evidence of clinical effectiveness in animal or in-vitro studies for use in emergency situations and meet emergency preparedness measures, such as the approval and distribution of a vaccine for the treatment and prevention of a pandemic influenza virus. |
Availability of products which only contain substantial evidence of clinical effectiveness in animal or in-vitro studies approved though a formal mechanism for use in emergency situations. |
Issue Analysis completed. Draft regulations in preparation. |
|
Food and Drug Regulations |
A new regulatory framework that is based on sound science and risk management is being developed. This will include regulations and guidance documents and will address many of the complex regulatory issues that are inadequately dealt with by the present regulations. |
A modern drug licensing framework for Canada that supports access to drugs while continuing to monitor the safety, efficacy, and quality of a drug throughout its lifecycle. |
Stakeholder consultation on the progressive licensing framework was conducted in Fall 2006. The Progressive Licensing website was launched in 2007. The website contains the concept document, brief discussion papers on topics relating to the regulation of drugs, and reports of workshops with stakeholders. |
|
Food and Drugs Act |
Reflect current safety standards for semen used in assisted conception. |
Regulations reflective of current safety standards for semen used in assisted conception. |
Significant policy analysis around the changes needed to the regulations has been completed. Discussion on how to best integrate the Semen Regulations under the Assisted Human Reproduction Act ongoing. |
|
Health Policy Branch |
|||
|
Regulations under the Assisted Human Reproduction Act |
New regulations will protect the health and safety of Canadians who use assisted human reproduction (AHR) procedures and ensure that AHR-related research, which may help find treatments for infertility and diseases, takes place in a controlled environment. Regulated activities include embryo research, clinical and laboratory practices and pre-implantation genetic diagnosis. The regulatory framework will also regulate reimbursement of expenditures, counselling, and health reporting information and include a licensing regime for AHR activities. |
Compliance with regulations measured through inspections, investigations, tracking of complaints and surveys. |
In the Spring and Fall of 2006, the Department consulted stakeholders to examine issues related to licensing, clinical and laboratory practices, health reporting information and counselling. A public consultation report was posted on Health Canada’s website entitled “Counselling Services Under the Assisted Human Reproduction Act” in March 2007. Further consultation documents and pre-publication in Canada Gazette, Part I are expected in Fall 2007 and Spring 2008. |
|
Regulations concerning Section 8 of the Assisted Human Reproduction Act |
Human reproductive material and embryos (including in vitro embryos) are used only with the donor’s written consent. The principle of free and informed consent is promoted and applied as a fundamental condition of the use of assisted human reproductive technologies. |
Compliance with regulations measured through inspections, investigations, tracking of complaints and surveys. |
Regulations were finalized to be registered (published in the Canada Gazette, Part II) June 27, 2007. These regulations will come into force in December 2007. |
|
Healthy Environments and Consumer Safety Branch |
|||
|
Tobacco Retail Promotion Regulations |
Reduced visibility of tobacco promotion at retail. Achievements will be measured through surveys at retail. |
Level of public awareness of tobacco product displays and other tobacco promotions at retail. |
Public consultation document released on proposals for further regulating the display and promotion of tobacco and related products at retail. |
|
Tobacco Promotion Regulations prohibiting “light” and “mild” descriptors |
Reduce confusion among smokers regarding these descriptors. Greater awareness that no class of cigarettes is a "safe" alternative. |
Number of smokers who believe that "light" and "mild" cigarettes are less harmful than regular cigarettes. |
Draft regulations and regulatory impact assessment were prepared. |
|
Introduction of New Tobacco Labelling Requirements |
Increased awareness of tobacco-related hazards. Increased knowledge of tobacco products and their emissions. |
Level of public awareness of health hazards related to tobacco use. Level of knowledge of tobacco products and their emissions. |
Creative concepts of health information messages and potential health warning messages for cigars and pipes, and smokeless tobacco products were developed and tested through public opinion research. This research supports evidence-based messaging in developing the new labelling requirements. |
|
Tobacco Advertising Regulations |
Increased awareness of tobacco-related hazards through mandating of new health warnings in advertising. Awareness will be measured through surveys. |
Level of public awareness of health hazards related to tobacco use. |
Potential health warning messages for advertisements of cigarettes, cigars and smokeless tobacco products were developed and tested through public opinion research. This research supports evidence-based messaging in developing health warnings for tobacco advertisements. |
|
Regulations Granting Authorities to New Classes of Practitioners under the Controlled Drugs and Substances Act |
Federal legislation will no longer restrict the professional practice of any health profession regulated by provincial or territorial (P/T) authorities, including practitioners of medicine, dentistry, veterinary medicine, paediatric medicine, midwifery, and nurse practitioners, with respect to the use of controlled substances in the treatment of their patients. |
Achievement will be measured by improved alignment of federal and P/T regulatory frameworks governing the appropriate use of controlled substances for medical purposes. |
Members of the Advisory Committee reviewed a draft policy framework during summer 2006 and a final draft policy framework to be issued in June 2007. A proposal for new regulations to be published for public consultation (pre-published in the Canada Gazette, Part I) on June 30, 2007 and final regulations are expected to be registered (published in the Canada Gazette, Part II) in late fall 2007. |
|
Pest Management Regulatory Agency(PMRA |
|||
|
Pest Control Products Incident Reporting Regulations |
Strengthened health and environmental protection from the use of pesticides through the establishment of a mandatory system for registrants to report incidents which will enable Health Canada's PMRA to identify trends in relation to products, uses and geography. |
A mandatory system in place in 2006 for the provision of pesticide incidents information by registrants, when they occur. |
A mandatory system for the provision of pesticide incidents information by registrants, when they occur, to come into effect on April 26, 2007. |
|
Pest Control Products Sales Information Reporting Regulations |
Strengthened health and environmental protection from the use of pesticides through the establishment of a mandatory system for registrants to report sales information about their pesticides which will enable Health Canada's Pest Management Regulatory Agency (PMRA) to more accurately identify trends in relation to products, uses and geography over time. |
A mandatory system in place for the provision of pesticide sales information by registrants in 2006. Provision of pesticide sales information by registrants in 2007. |
A mandatory system is in place since November 26, 2006, for the provision of pesticide sales information by registrants. Registrants are expected to provide pesticide sales information for year 2007 by the end of June 2008. |
Table 9: Details on Project Spending
(THOUSANDS OF DOLLARS)
|
Current Estimated Total Cost |
Actual 2004-05 |
Actual 2005-06 |
2006-07 |
||||
|---|---|---|---|---|---|---|---|
|
Main Estimates |
Planned Spending |
Total Authorities |
Actual |
||||
|
Program Activity |
Corporate Management |
||||||
|
The Way Forward: Planning |
N/A |
$2,388.9 |
$0 |
$0 |
|||
|
The Way Forward: Definition |
N/A |
$4,271.9 |
$0 |
$0 |
|||
|
The Way Forward: Implementation |
N/A |
$5,928.0 |
$14,354.2 |
$13,956.7 |
|||
|
The Way Forward: Close-out |
N/A |
$64.8 |
$382.5 |
$338.51 |
|||
|
The Way Forward: TOTAL |
$25,000* |
$12,653.6 |
$14,736.7 |
$14,295.2 |
|||
|
* - This funding covers both FY 05/06 and FY 06/07 |
|||||||
Notes:
1. The close-out costs of the "TWF" project completed in FY 2006-2007 is based on the formula calculated by the Project Manager.
Table 13 : Details on Transfer Payment Programs (TPPs) (2006-2007)
1. Table not to be included in printed DPR : include a listing of your completed TPP tables. Please include the following instruction to guide the reader to the tables available on-line:
"Supplementary information on Transfer Payment Programs can be found at http://www.tbs-sct.gc.ca/est-pre/estime.asp
Please complete the following table:
|
1) Name of Transfer Payment Program Grant for Nunavut Medical Travel Fund |
||||||
|
2) Start Date June 27, 2005 |
3) End Date March 2010 | |||||
|
4) Description To support the Nunavut medical travel fund |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved Initiatives to address the significant and immediate pressures facing the Yukon, Northwest Territories and Nunavut (the territories) in the area of medical travel expenditures |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
10.2 |
10.2 |
10.2 |
10.2 |
NIL |
|
14) Total Contributions |
|
|
|
|
|
|
|
15) Total PA |
|
10.2 |
10.2 |
10.2 |
10.2 |
NIL |
|
16) Comment(s) on Variance(s) N/A |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Grant to the Government of Yukon for the Territorial Health Access Fund and Operational Secretariat |
||||||
| 2) Start Date September 2005 | 3) End Date March 2010 | |||||
| 4) Description Grant to the Government of Yukon for the territorial Health Access Fund and Operational Secretariat | ||||||
| 5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians | ||||||
|
6) Results Achieved
|
||||||
| 7) Actual Spending 2004-05 | 8) Actual Spending 2005-06 |
9) Planned Spending 2006-07 | 10) Total Authorities 2006-07 |
11) Actual Spending 2006-07 |
12) Variance(s) Between 9 and 11 | |
|---|---|---|---|---|---|---|
| 13) Program Activity (PA) First Nations and Inuit Health | ||||||
| 14) Total Grants |
6.3 |
6.3 |
6.3 |
6.3 |
NIL |
|
| 14) Total Contributions | ||||||
| 14) Total Other Types of TPs | ||||||
| 15) Total PA |
6.3 |
6.3 |
6.3 |
6.3 |
NIL |
|
| 16) Comment(s) on Variance(s) | ||||||
| 17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: | ||||||
| Audit Findings and URL | ||||||
| Evaluation Findings and URL | ||||||
|
1) Name of Transfer Payment Program Payments to Indian bands, associations or groups for the control and provision of health services |
|||||||
|
2) Start Date June 1989 |
3) End Date 2006 |
||||||
|
4) Description This transfer payment represents payments made to Indian bands, associations or groups for the control and provision of health services. |
|||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
|||||||
|
6) Results Achieved Contributed to increased control and accountability by First Nations communities of health care services. |
|||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|
|---|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
|
14) Total Contributions |
205.2 |
202.3 |
217.9 |
204.3 |
204.3 |
13.6 |
|
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
|
15) Total PA |
205.2 |
202.3 |
217.9 |
204.3 |
204.3 |
13.6 |
|
|
16) Comment(s) on Variance(s) |
|||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
|||||||
|
Audit Findings and URL |
|||||||
|
Evaluation Findings and URL |
|||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Health Governance and Infrastructure Support (HG/IS) |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description Governance and Infrastructure Support to the First Nations and Inuit Health System |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canaians |
||||||
|
6) Results Achieved |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
76.9 |
167.6 |
95.0 |
87.8 |
79.8 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
76.9 |
167.6 |
95.0 |
87.8 |
79.8 |
|
16) Comment(s) on Variance(s) An amount of $19.5 was reprofiled to FY 2007-08. |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Community Programs |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description This transfer payment represents contributions made towards the Aboriginal Head Start On-Reserve Program. |
||||||
|
5) Strategic OutcomeBetter health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved Contributed to the developmental needs of FN/I children through activities that encouraged learning, healthy eating and hygiene and provided access to health services. |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
209.0 |
211.3 |
288.5 |
288.5 |
-77.2 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
209.0 |
211.3 |
288.5 |
288.5 |
-77.2 |
|
16) Comment(s) on Variance(s) variance explanation removed |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Health Facilities and Capital Program |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description This transfer payment represents contributions made on behalf of, or to, Indians or Inuit towards the cost of construction, extension or renovation of hospitals and other health care delivery facilities and institutions, as well as of hospital and health care equipment. |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved Provision of appropriate health care facilities for First Nations and Inuit clients on-reserve and modern, safe and secure functional office and living accommodations for staff. |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
41.4 |
51.9 |
41.7 |
41.7 |
10.2 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
41.4 |
51.9 |
41.7 |
41.7 |
10.2 |
|
16) Comment(s) on Variance(s) |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL Canada Prenatal Nutrition Program - First Nations and Inuit Health Branch - Evaluation Report 2006, 12 February 2007. http://www.hc-sc.gc.ca/ahc-asc/performance/eval/index_e.html |
||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Health Benefits |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description A limited range of medically necessary health-related goods and services which supplement those provided through other private or provincial/territorial health insurance plans is provided to registered Indians and recognized Inuit. Benefits include drugs, dental care, vision care, medical supplies and equipment, short-term crisis intervention mental health services, and transportation to access medical services not available on reserve or in the community of residence. |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
146.5 |
1 18.2 |
136.5 |
136.5 |
-18.3 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
146.5 |
1 18.2 |
136.5 |
136.5 |
-18.3 |
|
16) Comment(s) on Variance(s) |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Health Protection |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description Communicable Disease and Environmental Health and Research programs facilitate prepardness to implement measures in the control, management and containment of outbreaks of preventable diseases and improve management and control of environmental hazards. |
||||||
|
5) Strategic OutcomeBetter health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
15.9 |
9.7 |
29.0 |
29.0 |
-19.3 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
15.9 |
9.7 |
29.0 |
29.0 |
-19.3 |
|
16) Comment(s) on Variance(s) |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Contributions for First Nations and Inuit Primary Health Care |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description Primary Health Care services include emergency and acute care health services, Community primary health care services which include illness and injury prevention and health promotion activities. These programs also include: the First Nations and Inuit Home and Community Care; and the Oral Health Strategy. |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
134.0 |
119.7 |
95.1 |
91.7 |
28.0 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
134.0 |
119.7 |
95.1 |
91.7 |
28.0 |
|
16) Comment(s) on Variance(s) Funds were reallocated from within Branch to address identified funding pressures. |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
|
1) Name of Transfer Payment Program Contributions for Bigstone Non-Insured Health Benefits Pilot Project |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description Administration and delivery of benefits with Bigstone Health Commission to registered Indians and recognized Inuit. |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities |
11) Actual Spending |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
8.5 |
8.2 |
9.0 |
9.0 |
-.8 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
8.5 |
8.2 |
9.0 |
9.0 |
-.8 |
|
16) Comment(s) on Variance(s) |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL The Bigstone Cree Nation has taken on full administrative control of all but one of the non-insured health benefits after eight years. 2. It was felt that the pilot project was meeting the general goal of delivering non-insured health benefits in an effective, efficient and appropriate manner. 3. It was also felt that the NIHB pilot project was needed by the Bigstone Cree Nation and that it was very important that certain non-insured health benefits be administered locally. 4. The majority of respondents from the Bigstone Cree Nation's NIHB pilot project felt that administration had stayed the same or improved. 5. The Bigstone Cree Nation's pilot project was characterized by a strong, cooperative relationship between the Bigstone Health Commission and the FNIHB regional office. 6. A substantial part of the success of the Bigstone NIHB pilot project appears to have resulted from the support that the pilot project received from the regional FNIHB office, the expertise provided by FNIHB's knowledgeable personnel, and the support and commitment from the Bigstone Cree Nation's leadership. There was a large amount of satisfaction with the level of support provided to the pilot project by the regional FNIHB office. Both the Bigstone Cree Nation Pilot Project and FNIHB felt that the management structure had proved to be flexible, viable, efficient, effective and satisfactory. 7. Both the Bigstone Cree Nation and FNIHB were satisfied that the technological resources of the pilot project were sufficient to facilitate the administration of the benefits and both were somewhat satisfied with the adequacy of the human resources available to deliver benefits. FNIHB felt that additional staff training should be provided to cover staff turnover. The Bigstone Cree Nation Pilot Project noted that Bigstone had substantial human resources to draw on (such as the Chief and Band Council, the Bigstone Health Commission, consultants, and FNIHB staff), but had to develop their own local capacity. 8. It was felt that the pilot project had been affected by several external influences, including general inflation and cost/price increases, the shifting priorities and management focus of the Bigstone Health Commission, staffing changes at FNIHB, and the impact of new privacy laws which necessitated revised consent approaches. 9. Both the Bigstone Cree Nation Pilot Project and FNIHB felt that there was sufficient capacity to conduct the pilot. In addition, both felt that training needed to be ongoing in order to plan for staff replacements and program expansion and to develop new and/or enhanced skills and expertise in areas such as electronic record-keeping, utilization review and outcome evaluation. 10. It was suggested that the objectives of the pilot project should be reviewed to ensure that they are accurate and reasonable, the timelines for development and implementation should be extended substantially, and stronger monitoring/outcome evaluation processes should be developed for all First Nations health programs. |
||||||
|
1) Name of Transfer Payment Program Contributions to the Organization for the Advancement of Aboriginal People's Health (OAAPH) |
||||||
|
2) Start Date April 2005 |
3) End Date March 2010 | |||||
|
4) Description This transfer payment represents payments made to the Aboriginal Health Institute/Centre for the Advancement of Aboriginal Peoples' Health. |
||||||
|
5) Strategic Outcome Better health outcomes and reduction of health inequalities between FN/I and other Canadians |
||||||
|
6) Results Achieved The advancements in knowledge and the sharing of knowledge on aboriginal health contributed to continued empowerment of Aboriginal peoples. |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending 2005-06 |
9) Planned Spending 2006-07 |
10) Total Authorities 2006-07 |
11) Actual Spending 2006-07 |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) First Nations and Inuit Health |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
|
5.0 |
5.0 |
5.0 |
5.0 |
NIL |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
|
5.0 |
5.0 |
5.0 |
5.0 |
NIL |
|
16) Comment(s) on Variance(s) |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
|
Audit Findings and URL |
||||||
|
Evaluation Findings and URL |
||||||
Table 13 : Details on Transfer Payment Programs (TPPs)
|
1) Name of Transfer Payment Program The Alcohol and Drug Treatment and Rehabilitation Contribution Program |
||||||
|
2) Start Date |
3) End Date Ongoing |
|||||
|
4) DescriptionThe ADTR Contribution Program is an A-base component of the CDS which supports the federal government's efforts to reduce substance use and the harm associated with the abuse of alcohol and other drugs to individuals, families and communities. It provides cost-shared funding to participating provinces and territories through negotiated bi-lateral federal/provincial/territorial (FPT) contribution agreements. |
||||||
|
5) Strategic OutcomesIncreased access to and utilization of alcohol and drug treatment and rehabilitation services by women and youth. |
||||||
|
6) Results AchievedA review of the ADTR Program was completed in 2006-07. The review, along with other studies and consultations, concluded that systemic change was needed to move substance abuse treatment systems toward more evidence-informed practices, while increasing systems' capacity to evaluate practices for their efficiency and effectiveness. As a result, the ADTR Program will be refocused in 2008-09 to invest in activities that strengthen provincial/territorial substance abuse treatment systems. The refocused ADTR funding will invest in the following areas: 1) implementation of evidence-informed practices, 2) strengthening evaluation and performance measurement, and 3) linkage and exchange as it relates to 1) and 2). |
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending |
9) Planned Spending 2006-07 |
10) Total Authorities 2006-07 |
11) Actual Spending 2006-07 |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) - Healthy Environments and Consumer Safety |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
17.2 Includes redistribution spending |
14.2 Includes redistribution spending 13.3 |
14 |
13.3 |
13.2 |
.8 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
17.2 |
14.2 |
14 |
13.3 |
13.2 |
.8 |
|
16) Comment(s) on Variance(s)The program budget was reduced by $800K as part of the $1B budget reduction exercise. |
||||||
|
17) Audit/Evaluation Findings The CDS Interim evaluation did not examine the ADTR Program in any detail, therefore, no new evaluation findings are available. It should be noted that a performance measurement and evaluation plan are being developed in conjunction with the refocused ADTR Program. |
||||||
|
1) Name of Transfer Payment ProgramDrug Strategy Community Initiatives Fund (voted contributions dollars) |
||||||
|
2) Start Date |
3) End Date Ongoing |
|||||
|
4) DescriptionThe Drug Strategy Community Initiative Fund (DSCIF) was established under Canada's Drug Strategy (CDS) to provide support for community-based initiatives at the national, regional, provincial/territorial and local levels to facilitate community-based solutions to substance abuse problems and promote public awareness of substance abuse issues. Funding is provided in two main areas: Promotion and Prevention, and Harm Reduction. The Program is delivered through Health Canada's regional and national offices and the Northern region. *DSCIF will be transitioning to better reflect the new priorities under the National Anti-Drug Strategy. |
||||||
|
5) Strategic Outcomes
|
||||||
|
6) Results Achieved
|
||||||
|
|
7) Actual Spending 2004-05 |
8) Actual Spending 2005-06 |
9) Planned Spending 2006-07 |
10) Total Authorities 2006-07 |
11) Actual Spending 2006-07 |
12) Variance(s) Between 9 and 11 |
|---|---|---|---|---|---|---|
|
13) Program Activity (PA) -Healthy Environments and Consumer Safety |
|
|
|
|
|
|
|
14) Total Grants |
|
|
|
|
|
|
|
14) Total Contributions |
2.7 |
9.3 |
9.9 |
10.8 |
10.7 |
-.9 |
|
14) Total Other Types of TPs |
|
|
|
|
|
|
|
15) Total PA |
2.7 |
9.3 |
9.9 |
10.8 |
10.7 |
-.9 |
|
16) Comment(s) on Variance(s)Lapsed funds include monies that were unspent in the National component and the Regional components (with the exception of Ontario and Manitoba/ Saskatchewan) of the fund ($223,104.94). Monies lapsed due to reasons including: unspent funds at year-end under contribution agreements; monies not dedicated to specific projects; and monies that were unspent due to projects not receiving ministerial approval. The figures also represent the total allocation including the 182K reduction from the $1 billion reduction exercise and transfers from other departments. |
||||||
|
17) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or EvaluationThe DSCIF is evaluated as part of the Horizontal RMAF for Canada's Drug Strategy. The Interim Year-Two Risk-Based Evaluation of Canada's Drug Strategy shows that, with the exception of demand for funding exceeding supply, no potential risks identified for the DSCIF have arisen. The DSCIF successes identified in the report include: program implementation in a short time frame; strong partnerships with provincial/territorial partners; and, high number of applications. No audit of DSCIF has been conducted to date. Audit processes for funded projects have been undertaken, with no full audits required to date and two full audits have been conducted to date. (No URL available.) |
||||||
|
19) Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation The Contribution Program was evaluated in 2006-07 as part of the renewal of the Federal Tobacco Control Strategy. An audit of the FTCS Contribution Program was undertaken in 2003-04 as part of an overall Audit of Grants and Contributions Programs within the Healthy Environments and Consumer Safety Branch. |
||||||
Table 13
| Name of Transfer Payment Program: Named Grant to the Health Council of Canada |
||||||
| Start Date: September 1, 2004 |
End Date: Ongoing |
|||||
| Description: The mandate of the Health Council of Canada is to monitor and report annually on the implementation of the 2003 First Ministers Accord on Health Care Renewal, the 2004 Health Accord and on the health outcomes of Canadians. |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
| Results Achieved: The Health Council published Annual Reports in 2005, 2006 and 2007as well as a number of special reports which comment on the progress of health care renewal in Canada. The Council holds regular meetings and special events such as conferences and roundtables, and is supported by a small secretariat. |
||||||
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
|
Program Activity (PA) |
||||||
|
Total Grants |
4.7 |
3.1 |
10.0 |
6.0 |
4.6 |
5.4 |
|
Total Contributions |
||||||
|
Total Other Types of TPs |
||||||
|
Total PA |
4.7 |
3.1 |
10.0 |
6.0 |
4.6 |
5.4 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
| Name of Transfer Payment Program: Grant to the Canadian Agency for Drugs and Technology in Health (CADTH) |
||||||
| Start Date: April 1, 2005 |
End Date: Ongoing |
|||||
| Description: CADTH is an independent not-for-profit corporation established under the Canada Corporations Act, Part II. Its purpose is to facilitate the analysis, creation and dissemination of information concerning the effectiveness and cost of technologies and drugs, their impact on health and the appropriateness of their use. This named grant provides financial assistance to support CADTH's core business activities, namely: federal-provincial-territorial Common Drug Review (CDR), Health Technology Assessment (HTA) and Canadian Optimal Medication Prescribing and Utilization Service (COMPUS). |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
| Results Achieved: Evidence-based information that supports informed decisions on health technologies, including drugs, devices, medical and surgical procedures and health care systems. |
||||||
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
|
Program Activity (PA) |
||||||
|
Total Grants |
- |
7.4 |
17.4 |
17.4 |
17.0 |
0.4 |
|
Total Contributions |
||||||
|
Total Other Types of TPs |
||||||
|
Total PA |
- |
7.4 |
17.4 |
17.4 |
17.0 |
0.4 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
| Name of Transfer Payment Program: Grant to the Canadian Patient Safety Institute (CPSI) |
||||||
| Start Date: February 6, 2004 |
End Date: Ongoing |
|||||
| Description: CPSI is an independent not-for-profit corporation established under the Canada Corporations Act, Part II. Its purpose is to provide leadership and co-ordination in building a culture of patient safety and quality improvement throughout the Canadian health care system. CPSI works to: promote best practices; share information; offer advice to governments, stakeholders and the public on effective strategies to improve patient safety; and raise awareness with stakeholders, patients and the general public about patient safety. This named grant provides financial assistance to support CPSI's efforts to implement the provisions in the 2003 First Ministers' Accord towards improving health care quality by strengthening system co-ordination and national collaboration related to patient safety. |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
|
Results Achieved:
|
||||||
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
|
Program Activity (PA) |
||||||
|
Total Grants |
8.0 |
7.1 |
8.0 |
8.0 |
- |
8.0 |
|
Total Contributions |
||||||
|
Total Other Types of TPs |
||||||
|
Total PA |
8.0 |
7.1 |
8.0 |
8.0 |
- |
8.0 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
| Name of Transfer Payment Program: Health Care Strategies and Policy Contribution Program |
||||||
| Start Date: October 21, 2002 |
End Date: Ongoing |
|||||
| Description: The Program will provide policy analysis and advice to support the First Ministers’ commitment to a more accessible, high-quality, sustainable and accountable health system that will be adaptable to the needs of Canadians. The Program has evolved to include delivery of the Pan-Canadian Health Human Resource Strategy, the National Wait Times Initiative and the Internationally Educated Health Professionals Initiative. The Pan-Canadian Health Human Resources (HHR) Strategy aims to secure and maintain a stable and optimal health workforce that supports overall health care renewal and a decrease in wait times. The federal government committed $20 million annually to joint initiatives through the pan-Canadian HHR Strategy, including: Pan-Canadian Health Human Resource Planning; Interprofessional Education for Collaborative Patient Centred Practice; and Recruitment and Retention. The Internationally Educated Health Professionals Initiative (IEHPI) is also part of the HHR Strategy. It is designed to facilitate the integration of Internationally Educated Health Professionals (IEHPs) by reducing barriers to practice and assisting them in obtaining licensure within the Canadian health care workforce. This will result in an increased number of health providers to address current and looming workforce shortages and support the achievement of Patient Wait Times Guarantees. In the spring 2005 budget, the Canadian government committed $75 million over five years to support IEHPI. |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
|
Results Achieved:
|
||||||
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
|
Program Activity (PA): |
||||||
|
Total Grants |
9.4 |
23.2 |
29.1 |
27.5 |
26.7 |
2.4 |
|
Total Contributions |
||||||
|
Total Other Types of TPs |
||||||
|
Total PA |
9.4 |
23.2 |
29.1 |
27.5 |
26.7 |
2.4 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
| Name of Transfer Payment Program: Contributions for the Primary Health Care Transition Fund (PHCTF) |
||||||
| Start Date: June 13, 2001 |
End Date: March 31, 2007 |
|||||
| Description: The $800 million PHCTF was established in response to the First Ministers' Meeting 2000 commitment and recognition that improvements to primary health care are crucial to the renewal of the overall health care system. |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
|
Results Achieved:
|
||||||
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
|
Program Activity (PA) |
||||||
|
Total Grants |
||||||
|
Total Contributions |
210.8 |
184.8 |
75.6 |
74.1 |
72.7 |
2.9 |
|
Total Other Types of TPs |
||||||
|
Total PA |
210.8 |
184.8 |
75.6 |
74.1 |
72.7 |
2.9 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
| Name of Transfer Payment Program: Contribution Program to improve access to health services for official language minority communities |
||||||
| Start Date: June 18, 2003 |
End Date: Ongoing |
|||||
|
Description: The program funds networking initiatives as well as health care professional training and retention measures. Within this context, 14 contribution agreements have been signed with recipients in both communities: Société Santé en français and the Quebec Community Groups Network, which will receive $9.3 million and $4.7 million, respectively, over a five-year period to maintain and develop networks for facilitation and co-ordination of activities around health care issues. For training and retention, the Consortium national de formation en santé (10 post-secondary institutions) will receive $63 million over five years to ensure the availability of health care professionals who can work in French. As well, McGill University will receive $12 million to organize training activities to ensure the availability of health care professionals who can work in English in Quebec. |
||||||
| Strategic Outcomes: Strengthen knowledge base to address health and health care priorities. |
||||||
|
Results Achieved: (1) Community Networking Support The Formative Evaluation of the Community Networking Program for French-speaking minority communities was completed in May 2006 and presented the following observations:
English-Speaking Official Language Minority Communities The Preliminary Evaluation Report of the Health and Social Services Networking and Partnership Initiative (HSSNPI) of the Quebec Community Groups Network (QCGN) was released in October 2006. Outputs and short-term outcomes include the creation of networking units and the development of knowledge about the Anglophone communities. (2) Training and Retention of Health Professionals Data collected for the Formative Evaluation of the Health Care Training and Research Project of the Consortium national de formation en santé (CNFS) reveal an increasing number of candidates undertake health studies in French. The primary objective set forth by the ten member institutions of the Consortium was to accept 2500 new students over a five-year period, 2003-2008. As of the project's fourth year, 2135 candidates were registered in member institutions' health programs, which exceeds by 34% the goal set by the CNFS for 2006-2007. This finding suggests that the Consortium and its partners could achieve or surpass the original objective of 2500 new students. In the project’s third year of operation, the ten member institutions of the CNFS had generated 574 graduates, which constitutes a 55% increase over the project's objective for 2005-2006. The number of graduates to date suggests that the five-year target of 1200 graduates will be achieved. English-Speaking Official Language Minority Communities Over a three-year period — 2005-2006, 2006-2007, and 2007-2008 — four measures are being implemented under the McGill Training and Human Resources Development Project: a language training program ($4.8M), initiatives for labour market retention and distance support ($2.4M), seminars and conferences (($0.4M) and the creation of an innovation fund. McGill University is responsible for planning, implementation and evaluation of the Project ($4.0M). The Language Training Program trained health professionals in each region of Quebec through a significant number of course hours. The development of different teaching modes was met with varying levels of satisfaction among participants. It has been estimated that 1599 persons were trained in 2005-2006 and 1925 were trained in 2006-2007. |
||||||
|
Actual Spending 2004-05 |
Actual Spending 2005-06 |
Planned Spending 2006-07 |
Total Authorities 2006-07 |
Actual Spending 2006-07 |
Variance(s) Between Planned and Actual Spending |
|
|
Program Activity (PA) |
||||||
|
Total Grants |
||||||
|
Total Contributions |
14.8 |
21.0 |
23.0 |
24.1 |
24.1 |
-1.1 |
|
Total Other Types of TPs |
||||||
|
Total PA |
14.8 |
21.0 |
23.0 |
24.1 |
24.1 |
-1.1 |
|
Comment(s) on Variance(s): |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation: |
||||||
Table 13
|
Name of Transfer Payment Program |
||||||
|
Start Date |
End Date |
|||||
|
Description |
||||||
|
Strategic Outcomes |
||||||
|
Results Achieved |
||||||
|
|
Actual |
Actual |
Planned Spending |
Total Authorities |
Actual |
Variance(s) Between |
|
Program Activity (PA) |
|
|
|
|||
|
Total Grants |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
- |
|
Total Contributions |
|
|
|
|
|
|
|
Total Other Types of TPs |
|
|
|
|
|
|
|
Total PA |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
- |
|
Comment(s) on Variance(s) N/A |
||||||
|
Significant Audit and Evaluation Findings and URL (s) to Last Audit and / or Evaluation |
||||||
Table 11: Foundations - Conditional Grants
|
1) Name of Foundation: Canadian Health Services Research Foundation (CHSRF) |
||||||
|
2) Start Date: |
3) End Date: N/A |
4) Total Funding: |
||||
|
5) Description: Total federal funding for the CHSRF is as follows (CHSRF's programs also receive funding from other sources):
CHSRF's mission is to support evidence-informed decision-making in the organization, management and delivery of health services through funding research, building capacity and transferring knowledge. The Foundation's strategic objectives are to:
CHSRF's work contributes to Health Canada's aim of strengthening the knowledge base to address health and health care priorities. More specifically, CHSRF's programs further the development of health human resources, provide health managers with tools to improve primary and continuing care, and support nursing research from a health system perspective. |
||||||
|
6) Strategic Outcome: Strengthened knowledge base to address health and health care priorities. |
||||||
|
7) Summary of Results Achieved by the Recipient: Overall key achievements in 2006 include commissioning of research in priority theme areas; the establishment of the partnership and design for the 2007 triennial Listening for Direction III national consultation to identify priorities for health services and policy research (with an increase from a total of six to eight national partners, including Health Canada); the convening of a successful national symposium on primary health care research; the design and piloting of a Decision Support Syntheses Program; and the release of syntheses of research in priority areas such as nurse staffing and patient safety. In addition, an inaugural and successful National Forum on Knowledge Transfer and Exchange was held; an innovative dissemination campaign strategy for research was launched with a focus on nurse staffing and patient safety, and another on management of the health care workplace; and new series were launched to promote best practices in developing and sustaining networks (Network Notes), and to summarize key research articles in Foundation theme and activity areas (In the Know, Network Digest). With regard to Nursing Research Fund and EXTRA, the following were achieved: Nursing Research Fund: In 2006, a priority-setting discussion was held with 50 nursing stakeholders to set the Foundation's priorities for nursing research for the near future. A synthesis document, Staffing for Safety: A Synthesis of the Evidence on Nurse Staffing and Patient Safety, and a dissemination campaign targeting Canada's health and nursing decision makers, were launched. The Nurse Staffing and Patient Safety Knowledge Network - a collaborative venture between the Foundation, the Canadian Patient Safety Institute, the Canadian Nurses Association, and NurseONE, - held its first two face-to-face exchanges. EXTRA: The third cohort of 26 Fellows was selected in 2006. This brings the total number of Fellows enrolled in the program since its inception in 2004 to 76. The first cohort of Fellows graduated in 2006. Data shows that upon entry to EXTRA, 21% of this first cohort reported using research in their daily work "most or all of the time"; this had increased to 50% upon graduation from the program. Further, over 60% of these graduates reported "excellent" or "very good" ability to champion evidence-informed working decision making in their home organizations, whereas only 8% felt this way upon program entry. |
||||||
|
|
8) Actual |
9) Actual |
10) Planned |
11) Total |
12) Actual |
13) Variance |
|
14) Conditional |
0 |
0 |
0 |
0 |
0 |
0 |
| 15) Comments on Variances: The conditional grant was paid to CHSRF in installments previous to the 2004-2005 reporting period. | ||||||
|
16) Significant Audit and Evaluation Findings and URL to Last Audit &/or Evaluation: CHSRF commissioned its second International Review Panel Report in 2006. Significant findings include: CHSRF has developed Canadian health services research capacity, in general, and in nursing services, in particular. CHSRF has been an innovation incubator, developing innovative programs and activities in the health sector. The Foundation has been a successful knowledge broker between the research and decision making communities. CHSRF has become a national and international reference for those working on improving the use of research in decision-making in the health sector. The Panel noted that the CHSRF should consider expanding its role to become an enabler and support health organizations in implementing the results from research to improve health services delivery performance. CHSRF should also consider broadening the decision-making audience for its work to include clinical leaders, middle managers, and policy makers outside health care who have an impact on health. CHSRF should continue to develop partnerships and alliances, particularly with professional associations which can be useful intermediaries to reach the Foundation's target audiences. In addition, the Foundation commissioned two important internal controls reviews: a mini internal controls review of its payroll system, which cited significant improvements from the 2005 internal controls review, and the second compliance audit of its funded research programs and projects. The Foundation also developed an Internal Audit Plan for the period 2008‑2013 to ensure it assesses the various levels of controls throughout the organization. Both reports and the Internal Audit Plan are available upon request. |
||||||
| 17) URL to Foundation site: www.chsrf.ca | ||||||
| 18) URL to Foundation's Annual Report: http://chsrf.ca/other_documents/annual_reports/2006/index_e.php | ||||||
|
1) Name of Foundation: The Canadian Institute for Health Information (CIHI) |
||||||
|
2) Start Date: |
3) End Date: March 31, 2010 |
4) Total Funding: |
||||
|
5) Description
Roadmap III also included as an objective, improving data collection and reporting of health performance information, particularly as it related to progress made in the 2004 F/P/T Health Accord. |
||||||
|
6) Strategic Outcome: Strengthened knowledge base to address health system priorities |
||||||
|
7) Summary of Results Achieved: CIHI reported the following results achieved:
|
||||||
|
|
8) Actual |
9) Actual |
10) Planned |
11) Total |
12) Actual |
13) Variance |
|
14) Program Activity |
0 |
0 |
$19,740M |
$19,740M |
$19,740M |
$0 |
|
15) Comment on Variance: N/A |
||||||
|
16) Significant Audit and Evaluation Findings and URL to Last Audit &/or Evaluation: |
||||||
|
17) CIHI |
||||||
|
18) Annual Report |
||||||
Table 11: Foundations (Conditional Grants)
|
1) Name of Recipient: Canada Health Infoway Inc. ( Infoway ) |
||||||||
|
2) Start Date: |
3) End Date: |
4) Total Funding: |
||||||
|
5) Description Funding had previously been provided to Infoway on three occasions: $500 million in 2001 in support of the September 2000 First Ministers' Action Plan for Health System Renewal to strengthen a Canada-wide health infostructure, with the EHR as the key priority; $600 million in the First Ministers' Health Accord of February 2003, to accelerate implementation of the EHR and Telehealth; and $100 million as part of Budget 2004 to support the development of a pan-Canadian health surveillance system, with a particular focus on infectious disease. At the time of this Report, these areas of work remain Infoway's three main priorities, and results for each are described in Section 7, below. Infoway's collaborative approach, where the federal, provincial and territorial governments participate as equals, toward a common goal of modernizing Canada's health information system, is already reducing costs through coordinating effort and avoiding duplication. Joint work to develop and implement common standards to enhance interoperability, as well as on purchasing to help reduce costs, and sharing best practices as successful initiatives are replicated across the country appear to be working for the parties involved. |
||||||||
|
6) Strategic Outcome(s): Strengthened Knowledge Base to Address Health and Health Care Priorities |
||||||||
|
7) Summary of Results Achieved by the Recipient Electronic Health Records - Infoway's goal for EHRs, endorsed by all jurisdictions, is to put into place the basic elements of an interoperable electronic health record for 50% of Canadians by the end of 2009. By focussing mainly on implementation of seven key building blocks - drug, laboratory and diagnostic imaging systems; infostructure; registries; interoperable electronic health record systems and innovation and adoption initiatives, Canada Health Infoway achieved an interim goal of having 4% of the Canadian population with an interoperable EHR by March 31, 2006. As well, collaboration with and funding by Infoway has contributed to six provinces and territories having concrete plans to achieve 50% coverage of citizens in their jurisdictions with electronic health records by 2010, with five more P/T's indicating they will have many of the key iEHR components in place by that time Telehealth - Infoway has invested in/continued to invest in projects to expand and sustain telehealth initiatives, particularly in rural and remote communities, including Aboriginal and official language minority communities. It continues to work on linkages between telehealth and electronic health records systems, and the integration of telemedicine activities into mainstream healthcare service delivery. Telehealth strategic plans are now in place in most jurisdictions, with the goal of implementation of telehealth solutions by all jurisdictions by December 31, 2009. Health Surveillance - Infoway continued to fund the British Columbia Ministry of Health to manage the pan-Canadian Public Health Surveillance System project (Panorama) during 2006-07. Panorama is expected to comprise modules for Case Management, Outbreak, Immunization, Vaccine Inventory and Alerts. With vendor selection complete, work began on system design, with an anticipated release date of March 2008. |
||||||||
|
|
8) Actual Spending |
9) Actual Spending |
10) Planned Spending |
11) Total Authorities |
12) Actual Spending |
13) Variance Between 10) and 12) |
||
|
14) Program Activity |
$73.7M |
$117.8 M |
$385M |
$518M* |
$174.6M |
See note in #15 |
||
|
15) Comment(s) on Variance(s): While not "variances" per se, this explanation references the operational realities that produce differences in the amounts entered in the table above. As Infoway explains in its Annual Report, "(Infoway) program expenses are recognized on a milestone completion basis - when partners can match funds and deliver the projects. Many factors can have an impact on this aspect, including jurisdictional readiness, competing priorities, procurement procedures and vendor product readiness. Consequently, there is an inherent lag between project approvals and project expenditures... Overall, current expenditures tend to lag approvals by 24 to 36 months." |
||||||||
|
16) Significant Audit and Evaluation Findings and URL to Last Audit & / or Evaluation Independent performance evaluations will be done every five years. |
||||||||
| 17) URL to Recipient's Site: http://www.infoway-inforoute.ca/en/Home/home.aspx | ||||||||
| 18) URL to Annual Report: http://www.infoway-inforoute.ca/en/ResourceCenter/ResourceCenter.aspx | ||||||||
Table 15: Procurement and Contracting
In order to reduce the volume of printed material, this table is not to be included in the printed DPR.
Department
|
Points to Address |
Department's Input |
|---|---|
|
1. Role played by procurement and contracting in delivering programs |
Procurement and contracting are essential in terms of providing goods and services to the Department by contracting for services and procuring goods, particularly in the scientific and research disciplines. Collaboration, sharing of expertise and related information, as well as providing operational support, are key to ongoing policy and program delivery. |
|
2. Overview of how the department manages its contracting function |
Procurement and contracting are highly decentralized by branch and by region, with program managers designated with budgetary responsibilities as contracting authorities based on the Delegation of Financial Signing Authorities. Contract and procurement specialists provide support services directly to program branch operations in the National Capital Region (NCR) and in the Regions provide functional liaison through Regional Senior Financial Officer. Senior contract specialists have been co-located within each branch to provide improved oversight as part of the Contract and Requisition Control Committee and to promote procurement planning and the development of procurement strategies with the cooperation of program managers. Staffing is underway to provide similar capacity in every Region. Ongoing training is provided to cost centre managers and support staff who are involved in the contract process to ensure that informed decisions are made in compliance with regulations, policies and procedures. A quality assurance group, working in conjunction with internal audit, periodically assesses elements of contracting activity, including the use of acquisition cards, to ensure that the Department conforms to government policy on procurement and contracting. |
|
3. Progress and new initiatives enabling effective and efficient procurement practices |
In order to enhance the Department's capacity to provide improved service with respect to contracting and in response to the Accountability Act and the Procurement Reform Initiative, lead by PWGSC, additional resources were added and new initiatives undertaken. New positions were created and staff deployed to each branch within the NCR to provide consultation, advice and oversight as part of the Contract Requisition and Control Committee function, to develop procurement strategies for program managers and to liaise with all parties regarding the processing and administration of contracts. A similar network of staff will soon be in place to provide support to the regions. As part of its new Contract Management Framework, the Contract Requisition and Reporting System was introduced to the Department and can be described as a contract tracking, workflow and approval system. This system captures approximately 80 percent of contracting activities and continues to be expanded. It provides improved capacity to report on contract activity, to respond to Access to Information and Ministerial enquiries and also incorporates a review and approval workflow for contracts. Training was completely rolled out to the regions and the NCR. An action plan was developed specific to the integration of the Government of Canada Marketplace. Staff have participated in training for commodity panel participation and are actively involved in the commodity panel for temporary help services. The use of mandatory standing offers and the standing offer index offered by PWGSC were discussed and enhancements made, followed by a bulletin to all departmental employees requesting compliance in the use of mandatory standing offers. Annual Materiel Management Workshops are held to develop common understanding and working knowledge of contracting and materiel management issues. Presentations were given by managers from Materiel and Assets Management Directorate and Legal Services. New working tools were introduced to eliminate gaps in contract documentation formalizing certain contract activities. The Local Purchase Order for Services was created to contract for "basic" service delivery up to a maximum of $5,000 and the Memorandum of Agreement was created to include contractual clauses in agreements between Health Canada and other provincial/territorial or municipal administrations thereby replacing the Memorandum of Understanding where there is a transfer of funds. |
Table 17: Horizontal Initiative
|
Horizontal Initiative |
|||||||||||||||||||
|
1. Name of Horizontal Initiative: |
2. Name of Lead Department: |
||||||||||||||||||
|
3. Start Date of the Horizontal Initiative: |
4. End Date of the Horizontal Initiative: |
5. Total Federal Funding Allocation: |
|||||||||||||||||
|
6. Description of the Horizontal Initiative: Canada's Drug Strategy (CDS) was first introduced in 1987 to address substance use and abuse issues through coordinated activities by various federal departments, governments and non-governmental organizations. In 1992, following some initial successes in the areas of prevention and treatment, Phase II was launched with an emphasis on Driving While Impaired. During Phase II of the CDS, changing government priorities resulted in less than half of the funding being applied to the Strategy making it difficult to fully address complex issues related to both supply and demand reduction. CDS Renewed (approved by Cabinet in May 2003) is a comprehensive inter-departmental initiative to coordinate and enhance substance abuse programs, knowledge and partnerships in prevention, treatment, harm reduction and enforcement. For more information, please refer to http://www.hc-sc.gc.ca/ahc-asc/activit/strateg/drugs-drogues/index_e.html.[3] Budget 2007 announced the establishment of a National Anti-Drug Strategy (NADS) that will focus on prevention, treatment and enforcement activities. Future reporting will be based on these focus areas. |
|||||||||||||||||||
|
7. Shared Outcome(s): Improved Leadership - Setting directions and creating environments that support local action and national action integrally linked to nationally defined objectives and priorities; Enhanced knowledge generation and management - Providing strengthened capacity to improve evidence-based policy and decision making by promoting leading-edge research, statistical monitoring of drug trends and evaluation of program effectiveness; Enhanced partnerships and interventions - Discouraging substance abuse, targeting illegal conduct that threatens the safety and security of Canadians, and assisting those at risk from the effects of drugs by supporting partnerships and programs that focus on prevention, harm reduction, treatment and enforcement activities; Improved modernization of relevant legislation and drug policies - Ensuring that legal and policy approaches underpinning CDS are coherent with and support the Strategy, by reviewing legislation and regulations for responsiveness to current requirements. |
|||||||||||||||||||
|
8. Governance Structure(s):[4] Health Canada (HC) Health Canada is the federal lead for CDS. The Minister of Health is responsible for coordination across federal departments. Health Canada also partners with provinces and territories to provide national leadership and coordination, manages programs that reduce and prevent harm associated with controlled substances and participates in international fora in support of health-related supply and demand reduction activities. An Assistant Deputy Minister Interdepartmental Steering Committee is chaired by Health Canada. Working groups focusing on Communications, Research and Surveillance, Evaluation and Risk Management and Emerging Issues have been established to support decision making by the ADM Steering Committee and Health Canada provides a Secretariat to support these structures. Public Health Agency of Canada (PHAC) The Public Health Agency of Canada, through its Centre for Infectious Disease Prevention and Control (CIDPC) and its Fetal Alcohol Spectrum Disorder team, is responsible for conducting and disseminating research and surveillance information on public health indicators and illness related to substance use/abuse and injection drug use, as well as on the linkages between alcohol use during pregnancy and fetal alcohol spectrum disorder. Department of Public Safety Canada (PS)[5] The Department of Public Safety Canada is responsible for: a) coordinating the Public Safety Portfolio's drug control policies and initiatives to ensure that they are consistent with and complementary to the broader goals and objectives of CDS; and b) providing strategic advice to the Minister in fulfilment of the Minister's policy leadership role in policing and corrections. The Department also participates in international fora in support of law enforcement-related supply and demand reduction activities. Royal Canadian Mounted Police (RCMP) The RCMP offers a balanced approach addressing both supply and demand issues. RCMP officers investigate illegal drug activities and organized crime groups. They disrupt criminal activities and networks related to the supply of illicit drugs. They also deliver a number of drug awareness and prevention programs targeted at youth, Aboriginal communities, drug endangered children and parents. Additionally, they coordinate specialized training of police officers in Drug Recognition Expertise to detect drug impaired driving. Correctional Service Canada (CSC) CSC provides substance abuse treatment programs to federal offenders with substance abuse problems and controls the supply of illicit drugs in correctional facilities through various security measures. In addition, the Addictions Research Centre of CSC conducts research on substance abuse issues of importance to federal corrections and develops programs to address the substance abuse needs of offenders. Canada Border Services Agency (CBSA) The CBSA contributes to reducing the supply of controlled substances and illegal drugs through their detection and interception at Canadian ports/borders of entry. Department of Justice (DOJ) The Programs Branch of the Department of Justice, in collaboration with the Office of Demand Reduction of Health Canada, administers funding and monitors implementation and evaluation of the six federally-funded drug treatment courts. These courts are alternatives to traditional prosecution that integrate both criminal justice and drug treatment responses. The Federal Prosecution Service of the Department of Justice prosecutes drug cases. Drug cases comprise a significant part of the Prosecution's workload.[6] Department of Foreign Affairs and International Trade Canada (DFAIT) The Department of Foreign Affairs and International Trade Canada represents Canada, in cooperation and coordination with other interested CDS partners, on the international aspects of CDS. The Department and its network of overseas embassies and consulates, covers major international meetings (United Nations, G-8, international regional organizations), represents Canada at international processes (Dublin Group, Paris Pact, transnational organized crime instruments), as well as advocacy, diplomatic and technical assistance activities with bilateral partners. The Department manages Canada's umbrella contribution agreements to the UN Office on Drugs and Crime (UNODC) and the Organization of American States' Inter-American Drug Abuse Control Commission (CICAD), and other drug cooperation projects under the Public Safety Envelope of its Human Security Program. |
|||||||||||||||||||
|
9. Federal Partners Involved in each Program |
10. Names of Programs |
11. Total Allocation |
12. Forecasted Spending for 2006‑07 |
13. Actual Spending in 2006‑07 |
|||||||||||||||
|
Health Canada |
Promotion / Prevention and Public and Professional |
$ 14.83 M |
$ 5.85 M |
$ 5.85 M |
|||||||||||||||
| RCMP | Education / Training | $ 18.46 M | $ 5.26 M | $ 5.26 M | |||||||||||||||
| Canadian Centre on Substance Abuse (CCSA) | Programs / Activities | $ 7.00 M | $ 2.45 M | $ 2.45 M | |||||||||||||||
| $ 40.29 M | $13.56 M | $ 13.56 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007: Prevention activities under CDS include measures to educate people about the dangers of drug abuse and help them adopt healthy behaviours.
|
|||||||||||||||||||
|
Health Canada |
Treatment and Rehabilitation Programs / Activities |
$ 347.20 M[8] |
$ 87.10 M |
$85.3 M[9] |
|||||||||||||||
| Correctional Service Canada | $ 69.80 M | $ 19.10 M | $19.20 M | ||||||||||||||||
| Department of Justice | $ 10.20 M | $ 3.80 M | $2.30 M[10] | ||||||||||||||||
| $ 427.20 M | $110.00 M | $106.80 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007: Treatment-related activities under CDS focus primarily on provision of treatment programs and services to targeted populations such as offenders, women and youth, and First Nations communities.
|
|||||||||||||||||||
|
Health Canada |
Research and Surveillance Programs / Activities |
$ 42.46 M |
$ 11.04 M[12] |
$ 9.63 M[13] |
|||||||||||||||
| PHAC | $ 4.50 M | $ 1.50 M | $ 1.50 M | ||||||||||||||||
| CSC | $ 8.10 M | $ 2.00 M | $ 2.10 M | ||||||||||||||||
| CCSA | $ 6.40 M | $ 1.30 M | $ 1.30 M | ||||||||||||||||
| $ 61.46 M | $ 15.84 M | $ 14.53 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007: CDS invests in the generation of new knowledge and improved knowledge management to enhance capacity to address existing and emerging substance abuse/use trends by contributing to the development and sharing of knowledge on more evidence-based approaches to substance abuse.
|
|||||||||||||||||||
|
Health Canada |
Contributions Programs |
$ 33.88 M |
$ 14.78 M |
$12.90 M[14] |
|||||||||||||||
| Foreign Affairs Canada | $ 10.8 M | $ 2.90 M | $ 2.90 M | ||||||||||||||||
| Public Safety | $ .51 M | $ .10 M | $ .10 M | ||||||||||||||||
| $ 45.19 M | $ 17.78 M | $ 15.90 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007:
|
|||||||||||||||||||
|
Health Canada |
Coordination and Collaboration Programs/Activities |
$ 13.16 M |
$3.37 M |
$ 3.37 M |
|||||||||||||||
| Public Safety | $ 2.51 M | $ .63 M | $ .45 M[15] | ||||||||||||||||
| $ 15.67 M | $4 M | $ 3.82 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007:
|
|||||||||||||||||||
|
Health Canada |
Policy and Legislative Review and Development Programs/ Activities |
$ 5.70 M |
$1.55 M |
$ 1.55 M |
|||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007:
|
|||||||||||||||||||
|
Health Canada |
Enforcement Programs/ Activities |
$ 55.74 M |
$ 14.26 M |
$ 14.26 M |
|||||||||||||||
| RCMP | $ 310.01 M | $ 79.07 M | $ 79.07 M | ||||||||||||||||
| Canada Border Services | $ 327.60 M | $ 81.90 M | $ 81.90 M | ||||||||||||||||
| Correctional Service Canada | $ 23.90 M | $ 5.90 M | $ 5.80 M | ||||||||||||||||
| Justice | $ 255.44 M | $ 64.50 M | $ 60.24 M | ||||||||||||||||
| $ 972.69 M | $ 245.63 M | $ 241.27 M | |||||||||||||||||
|
Planned Results for 2006-2007:
|
|||||||||||||||||||
|
Achieved Results for 2006-2007: Enforcement activities under CDS include measures to halt the unlawful import, export, production, distribution and possession of controlled substances.
|
|||||||||||||||||||
|
Total $1,568.2 M |
Total $408.36 M |
Total $397.43 M |
|||||||||||||||||
|
16. Comments on Variances: Comments on variances are provided in footnotes at the bottom of each relevant section. |
|||||||||||||||||||
|
17. Results Achieved by Non-federal Partners: |
|||||||||||||||||||
|
18. Contact Information: Colleen Ryan Manager, CDS Evaluation, Risk Management and Reporting (613) 957-2867 |
19. Approved by: Andrew Adams |
20. Date Approved: |
|||||||||||||||||
1 CDS was initiated in 1987 and has undergone a number of reiterations in the past 17 years. CDS Renewed was approved in May 2003.
2 The financials presented reflect a start date of May 2003 and an end date of 2006-2007. The funding allocation during this four year period is inclusive of both the enhanced funding received under CDS Renewed ($245 million over five years and ongoing) and A-base funding pertaining to activities undertaken in demand and supply reduction.
3 In light of the announcement of a National Anti-Drug Strategy in Budget 2007, the CDS website, which is a component of the Health Canada website, is in the process of being updated.
4 The governance structure may change under the NADS. Any changes will be discussed in the 2007-08 DPR.
5 The Department of Public Safety and Emergency Preparedness Canada has been changed to Public Safety Canada.
6 On December 12, 2006, the Federal Prosecution Service of Justice Canada became the new federal Public Prosecutions Service of Canada (PPSC) as a result of the Federal Accountability Act and related enactment of the Director of Public Prosecution Act. The PPSC is now independent of the Department of Justice and reports to Parliament through the Attorney General of Canada.
7 The CDS website was launched in August 2005. As such, statistics from last year were only available for August to December 2005.
8 During 2006-07, this total was revisited and .68 M was moved from Treatment and Rehabilitation Programs to Contributions Programs to reflect Health Canada’s component of the Drug Treatment Courts.
9 $800 K was removed from the original $14 M budget under the Health Canada component as part of the $1 B Expenditure Review.
10 The difference of $1.5 M between forecasted and actual spending reflects the amount allocated to Health Canada’s contribution to the Drug Treatment Court Funding Program.
11 Completions include those offenders who remain until the end of the program, both successful completions and those who attend all sessions.
12 The identified amount represents a combination of the enhanced funding provided to Health Canada under the renewal of CDS in addition to funds already being invested in research and surveillance activities prior to the renewal and prior to the separation of the Public Health Agency of Canada from Health Canada.
13 The difference of $1.41 M between forecasted and actual spending is due to a lapse of funds related to operational delays in delivery of research and surveillance activities, including approximately $500 K in lapsed funds related to delays in the contracting process with PWGSC for the Canadian Alcohol and Drug Monitoring Survey and approximately $900 K for projects that were postponed or cancelled due to changing priorities and delays in the approval process at the provincial/territorial level; monies unspent due to publications not receiving Ministerial approval; as well as monies not being transferred to other agencies due to delays in production of their reports.
14 Health Canada figures represent total allocation amounts for the Drug Treatment Court Funding Program ($2.78 M) and the Drug Strategy Community Initiatives Fund ($12 M). The variance is due to a lapse in funding for DSCIF in the amount of $223,104 due to unspent funds at year-end under contribution agreements; monies not dedicated to specific projects; monies unspent due to projects not receiving Ministerial approval as well as the $182 K reduction from the $1 B Expenditure Review and transfers from other departments. The variance for DTCFP was $1.5 M due to a $1.5 M contribution to the Expenditure Review.
15 Due to unusual operational delays in obtaining required information and approval related to planned contributions, a percentage of the contribution funding could not be expended. In addition, human resource-related issues resulted in less than complete use of operational funds.
Table 17 Horizontal Initiative
| 1. Federal Strategy on Early Childhood Development (ECD) for First Nations and Other Aboriginal Children | 2. Health Canada, First Nations and Inuit Health Branch | |||||
| 3. Start Date of the Horizontal Initiative: ECD - October 2002 Early Learning and Child Care (ELCC) - December 2004 |
4. End Date of the Horizontal Initiative: ECD - 2006-07 and ongoing ELCC - 2007-08 and ongoing |
5. Total Federal Funding Allocation: ECD in 2002 - $320 M over 5 years ($65 M ongoing) to enhance and expand various federal ECD programs ELCC in 2004 - $45 M over three years ($14 M ongoing) to increase integration, coordination, access and quality of two ECD/ELCC programs |
||||
|
6. Description of the Horizontal Initiative: The Federal Strategy on ECD for First Nations and Other Aboriginal Children was announced on October 31, 2002. The Strategy provides $320 million over five years to: improve and expand existing ECD programs and services for Aboriginal children; expand ECD capacity and networks; introduce new research initiatives to improve understanding of how Aboriginal children are doing; and work towards the development of a "single window" approach to ensure better integration and coordination of federal Aboriginal ECD programming. In December 2004, as the first phase of a "single window," Cabinet approved an additional $45 million over three years ($14 million ongoing) to improve integration and coordination of two ECD programs, (Aboriginal Head Start On-Reserve and the First Nations and Inuit Child Care Initiative), beginning in 2005-2006. The objectives of these funds are to increase access to and improve the quality of ELCC programming for First Nations children on-reserve, and improve integration and coordination between the two programs through joint planning, joint training and co-location. Joint planning will also include Indian and Northern Affairs Canada (INAC) funded child care programs. |
||||||
|
7. Shared Outcome(s): The funding, approved in December 2004, to enhance child care and work towards the first phase of a "single window", complements funding released to provinces and territories under the March 2003 Multilateral Framework for Early Learning and Child Care to improve access to early learning and child care programs and services. |
||||||
| 8. Governance Structure(s): Interdepartmental ECD Assistant Deputy Minister Steering Committee; Interdepartmental ECD Working Group. | ||||||
|
9. Federal Partners |
10. Names of Programs for the Federal Partners |
11. Total Allocation |
12. Planned Spending for 2006-07i |
13. Actual Spending in 2006-07 |
14. Planned Results for 2006-07 |
15. Results Achieved in 2006-07 |
|---|---|---|---|---|---|---|
|
a) Health Canada (HC) |
(a) Aboriginal Head Start On-Reserve (AHSOR) |
$21.5 M $7.0 M |
$20.6M 1 $8.3 M |
$26 M |
Program expansion and enhancement |
See details below |
|
$15 M |
$14.9 M |
$14.3 M |
Program expansion and enhancement |
See details below |
||
|
(c) Building Capacity and Networks |
$1.02 M |
$.95 M |
$.66 M |
Building capacity and networks |
See details below |
|
|
(d) Horizontal Training |
$1.3 M |
$1.3 M |
$1.3 M |
|
See details below |
|
|
b) Public Health Agency of Canada (PHAC) |
(a) Aboriginal Head Start in Urban and Northern Communities |
$ 12.6 M |
$12.6 M |
$12.6 M |
Program expansion and enhancement |
See details below |
|
(b) Building Capacity and Networks |
$500 K |
$500 K |
$500 K |
Program expansion and enhancement |
See details below |
|
|
c) Human Resources and Social Development Canada (HRSDC) |
(a) First Nations and Inuit Child Care Initiative (FNICCI) |
$9.14 M $7 M |
$9.14 M $7 M |
$16.14 M |
Program expansion and enhancement |
See details below |
|
(b) Aboriginal Children's Survey |
$3.44 M ongoing |
$6 M2 |
$6 M (est)2 |
Consultations, finalize content development, collection, data processing and promotional activities development. |
See details below |
|
|
(c) Understanding the Early Years - Aboriginal component |
$700 K |
$700 K |
$03 |
Understanding the Early Years Call for Proposals to encourage applications from Aboriginal communities |
Held consultations with national First Nations, Metis and Inuit organizations |
|
|
d) Indian Affairs and Northern Development
(INAC)
|
(a) "Single Window" Work |
$0.879 M |
$0.879M |
$0.879 M |
Better coordination and integration |
See details below |
|
(b) Building Capacity and Networks |
$0.131 M | $0.131M | $0.131 M |
|
See details below |
|
|
|
|
Total $80.21M |
Total $82.27M |
Total |
|
|
|
16. Comments on Variances 2 Aboriginal Children's Survey: 3 Understanding the Early Years (UEY) - Aboriginal component: |
||||||
| 17. Results Achieved by Non-federal Partners - see details below | ||||||
| 18. Contact Information Geoffrey Gurd, Manager, ECD, First Nations and Inuit Health Branch, Health Canada Postal Locator 1920D Tunney's Pasture, Ottawa Telephone: (613) 952-5064 Fax: (613) 952-5244 |
19. Approved by Kathy Langlois Director General Community Programs Directorate First Nations and Inuit Health Branch |
20. Date Approved | ||||
i The allocations, planned spending and actual spending figures (above) include only incremental funding provided under two initiatives: the 2002 Federal Strategy on ECD for First Nations and Other Aboriginal Children and the 2004 initiative to enhance child care and working towards the first phase of the "single window." These figures do not include base allocations to programs.
Aboriginal Head Start On-Reserve (AHSOR):
Details on results achieved in 2006-2007 are not available, as the AHSOR National Office will not receive the AHSOR regional progress reports until November 2007. In 2005-2006 reports, regions committed to some of the following activities:
- promote integration and cooperation between AHSOR and other early childhood programs both at the community level and at the regional level;
- continue to organize training in different areas to help maintain and improve the quality of the AHSOR program;
- improve reporting and communications between HC and communities;
- continue to develop and improve on outreach/home visiting programs.
The following can be said regarding 2006-2007:
- Approximately 9,000 children participated in AHS programs.
- Integration of sites and cooperation among different programs such as Health Canada's AHSOR, HRSDC's FNICCI, INAC's daycares in Ontario and Alberta, as well as local and provincial programs continued to take place at community and regional levels.
- AHSOR capital infrastructure was enhanced through the support of capital projects.
- AHSOR funding was expanded to a further 96 First Nations communities in Ontario. The majority of funding was used for program preparation and development including training and minor capital.
- Training was provided in some regions for AHSOR outreach and home visit workers for unserved and underserved communities
Fetal Alcohol Spectrum Disorder - First Nations and Inuit Component:
Building Capacity and Networks:
As part of the 2002 Federal Strategy's capacity-building component, Health Canada funding is provided annually to five national Aboriginal organizations: the Assembly of First Nations (AFN), Inuit Tapiriit Kanatami, Congress of Aboriginal Peoples, Métis National Council, and Native Women's Association of Canada. As well, Indian and Northern Affairs Canada is providing annual
funding to Pauktuutit Inuit Women of Canada. This funding enabled these national Aboriginal organizations to contribute through strategic planning and capacity building in their own organizations.
Funding also continued to support development of an Aboriginal service providers' network, called the Aboriginal Children's Circle of Early Learning (ACCEL). Departments were assessing the effectiveness of the network, and started to explore alternate ways of meeting community needs for networking and providing access to program resources. Culturally specific ECD materials were developed by the Inuit Tapiriit Kanatami and the Métis National Council for inclusion on the ACCEL website.
Most of this funding goes to regions to support training for ECD workers in AHSOR and FNICCI sites. A working group has been established with representation from AFN, INAC, HC and HRSDC and is developing a laddered ECD training strategy. This will lead to culturally appropriate certification of providers of ELCC programming for First Nations children living on-reserve and supports coordination between AHSOR, FNICCI and INAC funded daycares in Alberta and Ontario. A scan of available ECD training and providers has been completed.
Aboriginal Head Start in Urban and Northern Communities (AHSUNC):
- Improve access for children: 130 sites serving 4,500 children in 2007.
- Enhance special needs training, new resources
- Strengthen community capacity and support networks: According to the 2003-2005 Impact Evaluation, AHS has increased social networking among parents and staff. In some communities, the networking is linked to the community having increased cultural activities. Additionally, the volunteer and employment opportunities have led to more people in the community contributing to the local economy.
- Optimal development of children's school readiness: The 2005 Impact Evaluation indicates that Kindergarten teachers report high levels of personal and social development in AHSUNC children and that school readiness findings are comparable to outcomes reported in similar early intervention studies.
- Parental involvement, social support, health promotion, nutrition: From the 2005 Impact Evaluation, parents of AHSUNC three and four year olds note positive changes in their children's physical development, health and nutritional practices and personal/social development. Positive changes are noted in family nutrition and health practices, in particular with the serving of more nutritious food in the home and children improving dental and other hygiene practices.
- Impact Evaluation feedback to communities was undertaken.
HRSDC collaborated with other departments on Aboriginal Early Childhood Development to explore options, and develop a "single window" service delivery approach while supporting quality child care programs on-reserve and in the North. Program expansion and enhancement took place through joint planning, training and capital.
Aboriginal Children's Survey (ACS):
Understanding the Early Years (UEY) - Aboriginal component:
In preparation for the 2006 UEY Call for Proposals and fielding of the ACS, UEY staff consulted with federal colleagues in HRSDC, INAC, PHAC and HC to discuss a strategy for encouraging proposals from Aboriginal communities. A communications strategy was launched with key national organizations: Assembly of First Nations, Inuit Tapiriit Kanatami, Pauktuutit, Métis National
Council and Native Women's Association of Canada, Congress of Aboriginal Peoples and the National Association of Friendship Centres.
INAC began work, in conjunction with federal partners, on Single Window Service Delivery Demonstration Projects which would explore integration and coordination of ECD Programs within existing authorities. Over 50 First Nations communities expressed interest in participating and 17 were recommended for these projects. Those selected will be testing in 2007-2008 either streamlined reporting and a single funding mechanism; streamlined reporting, a single funding mechanism and community coordination/integration and development; or streamlined reporting only. Results will enable departments to assess which approach best increases accountability, provides community-level flexibility, streamlines financial reporting, improves coordination, and enhances the quality of ECD programming. In 2006, the ECD Working Group held meetings with national Aboriginal organizations to further work on the Single Window. In March 2007, Orientation and Training sessions on these demonstration projects were held for members from each participating community. Sessions were also held with regional employees from the federal departments involved to discuss their role in these activities.
Table 17 : Horizontal Initiative
| 1. Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products | ||||||
| 2. Name of Lead Department: Pest Management Regulatory Agency (PMRA) - Health Canada (HC) | ||||||
| 3. Start Date: 2002–2003 | 4. End Date: 2008–2009 | |||||
|
6. Description of the Horizontal Initiative The initiative is a part of the federal government’s commitments as outlined in the Treasury Board submission Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products. The submission and its associated Results-based Management and Accountability Framework (RMAF) describe the integrated approach by which initiatives will be measured, managed and reported throughout their life cycle. An important element of the commitments made is that stakeholders and the public will be kept informed through a transparent management system. Participating departments will work together for shared outcomes; measure performance on delivery; and review progress achieved. This initiative incorporates six federal government partners to increase public and stakeholder confidence in the pesticide regulatory system, to protect health and environment, and to increase the competitiveness of the agri-food and forestry sectors. Research and monitoring in the area of pesticides is being coordinated with their regulation. The presence and effects of pesticides in the environment, in marine and freshwater ecosystems, and in the forest environment are being monitored. The initiative enhances monitoring and enforcement of pesticide residue limits in foods, in feed, of pesticide residues in fertilizers, and pesticide guarantee verification for fertilizer-pesticide combinations. Reduced-risk pesticides and biological pesticides for forestry are being developed and their use facilitated. Commodity-based risk reduction strategies for the agriculture and agri-food sector are being developed and implemented. Programs improving access to agricultural minor-use pesticides and reduced-risk pesticides for agricultural use are being established. Research to support introduction of minor-use pesticides that pose a reduced risk to the environment is being conducted. A reporting system to track adverse effects of pesticides has been developed, and information will be collected and recorded. Collectively, this work is being conducted to achieve public confidence in increased conservation and protection of human health and the environment while contributing to the competitiveness of Canada ’s agricultural sector. The information in this table has been organized along three main themes: 1. Research and Monitoring, carried out by Agriculture and Agri-food Canada (AAFC), the Canadian Food Inspection Agency (CFIA), the Department of Fisheries and Ocean (DFO), Environment Canada (EC), Health Canada’s PMRA, and Natural Resources Canada (NRCan) 2. Developing and Implementing Commodity Specific Risk Reduction Strategies, carried out by AAFC and HC’s PMRA 3. Generation of Data to Support Registration of Reduced-Risk and Minor-Use Pesticides for the Agricultural and Agri-food Sector and Reduced-Risk Pesticides and Biopesticides for Forestry, carried out by AAFC, HC’s PMRA and NRCan |
||||||
|
7. Shared Outcomes: Immediate Outcomes:
Intermediate Outcomes:
Final Outcome: Increased public and stakeholder confidence in pesticide regulation, protected health and environment as well as increased competitiveness of the agri-food and forestry sectors |
||||||
|
8. Governance Structures:
|
||||||
|
9. Federal Partners |
10. Names of Programs |
11. Total Allocation |
12. Planned Spending for 2006–2007 |
13. Actual Spending for 2006–2007 |
14. Expected Results for 2006–2007 |
15. Actual Results for 2006–2007 |
| AAFC, CFIA, DFO, EC, HC (PMRA), NRCan | I. RESEARCH AND MONITORING | |||||
|
1. AAFC |
(a) Conducting research to support introduction of minor-use pesticides that pose a reduced risk to the environment.
|
$8.0 M
|
$2.0 M |
$ .04 M AL .01 M EBP 1.50 M NPO
|
Following evaluation of research projects, continued funding for some as appropriate. Final reports and next steps for implementation of research results under way for projects completed in March 2006. Initiation (in April,2006) of approximately 20 projects in Minor-Use Research and Biopesticide Initiatives as a result of November 2005 Project Call. Results of one year of research work on these projects to be reported upon (April 2007). Research planning, coordination continued with MOU Research Working Group. |
Continued funding approved for 10 of 12 projects evaluated during February 2006 Evaluation Workshop for fourth (final) year. Two projects completed as of April 2006 resulted in: 1) Integrated Pest Management (IPM) plan for management of leek moth, including submissions for registration of reduced-risk products Btk and spinosad and, 2) Identification of RR herbicide options for use in special crops, and data to support registration of sulfentrazone and carfentrazone-ethyl. Final reports for 10 ongoing projects due Q1 2007-2008. As a result of the November 2005 Project Call for Proposals, new projects were selected in 2006 for funding (under Minor-Use Research, Biopesticides, and Screening Trials Initiatives). A total of 30 projects were funded (20 new, 10 ongoing; the remaining project was selected in March 2006 and begins April 2007); 97% (29 of 30 projects) have met milestones to the end of March 2007. Research priorities and results were shared at two meetings of AAFC/PMRA Minor-Use Research Working Group (July, November) and one meeting of 5NR Pesticide Working Group (October) and the 5NR Environmental Assessment Workshop (February 2007). Extra activity 1: Biopesticides work with registrants. Submission of data package to support Category A registration of BlightBan A506 to PMRA. Submission of a bio-fungicide package to the registrant anticipated before end of May 2007. Submission of data package to registrant to support label expansion of Surround. Assistance provided to registrant for preparation of data package to support Category A submission of Met 52. Also as a result of promotional activities with U.S. and Canadian biopesticide companies, there is new interest in Canadian registrations. Several companies have made new submissions directly to PMRA. Extra Activity 2: Selection of projects where pest solutions don’t appear to exist. Developed and piloted a proposal with Minor- Use Program personnel, resulting in funding of two projects to screen for reduced-risk solutions (green mould in mushrooms and weeds in ginseng). |
|
2. CFIA |
(b) Enhanced monitoring and enforcement of pesticide residue limits in food and feed. |
$2.95 M |
$0.25 M |
$0.25 M |
Identify food commodities consumed by targeted subgroup (children). Lab testing of approximately 1,500 samples per year. Follow-up inspections for non-compliant test sample results. Publish annual report of findings of National Chemical Residues Monitoring Program (NCRMP). Food recalls, as required, for risk mitigation and removal of hazardous foods from marketplace. |
Program targeted traditional and organically labelled baby foods. Method used in analysis of samples was perfected by CFIA as a result of funding received in this initiative. Increased sensitivity was achieved in these analyses. |
|
2. CFIA cont’d |
(c) Enhanced monitoring and enforcement of pesticide residues in fertilizers and pesticide guarantee verification in fertilizer-pesticide combinations. |
$2.15 M |
$0.25 M |
$156 K lapsed funding to be used in next fiscal year |
Develop monitoring and surveillance policies and processes to guide and advise operational staff on fertilizer-pesticide combinations and pesticide contaminated fertilizers. Increase interaction with PMRA to obtain the most up-to-date pesticide safety and labelling information. Update the Compendium of Fertilizer-Use Pesticides, which contains information regarding registration, guarantees and proper labelling. Work to develop regulatory changes to facilitate updating of the Compendium, and, if successful, provide updates more regularly to producers of mixtures and to CFIA’s inspection staff. Advise CFIA Operations on appropriate follow-up procedures and recommendations regarding the significance of sample analytical results. Sample fertilizer-pesticide combinations to verify guarantees. Sample fertilizers suspected to be contaminated with pesticides. Verify fertilizer-pesticide labels. Conduct investigation and compliance activities (anticipated based on sampling and inspection frequencies). Analyze samples submitted by inspectors. |
Inspection Memorandum I-4-93, a document identifying inspection activities and sample quotas for the year, was provided to inspection staff. To facilitate label verification in the field and maintain consistency, a list of all registered fertilizer-pesticides and labels was updated and distributed to inspectors. Inspectors were guided on appropriate non-compliance follow-up when needed. The pesticide guarantee verification program has been redesigned, with the help of stakeholders, to improve compliance rate. CFIA’s tolerance for pesticide residues in fertilizers was reviewed and amended. Enforcement procedures in response to non-compliance were developed through a National Training Initiative to promote consistency in enforcement actions across Canada. CFIA and PMRA are jointly working to develop sub-agreements to an existing Memorandum of Understanding on regulation and/or registration of products under the Fertilizers Act and Pest Control Products Act, as well as a process to increase communication between the two agencies. CFIA is participating in the Building Public Confidence TB Initiative Evaluation Working Group. CFIA is participating in the 6NR Pesticides and Pest Management Working Group. The 3rd edition of the CFUP is pending in Canada Gazette, Part II. CFIA is looking into regulatory changes and expedited mechanisms to allow for more frequent updates. A new format is being created to facilitate public availability, and updates were distributed. Inspectors took 140 samples of fertilizer-pesticides for pesticide guarantee verification, and 80 samples of fertilizer products for the presence of pesticide residues. This is more than a three-fold increase for both programs over the average number of samples taken from 2000-01 to 2002-03. Follow-up actions on 49 non-compliant samples included warning letters, product detentions and investigations. Inspectors reviewed 137 fertilizer-pesticide labels for compliance with the Fertilizers Act and Regulations. Number of samples analyzed was 131 for pesticide guarantee verification and 63 for pesticide contamination audit. Analytical methodologies for the guarantee verification of 32 pesticides were reviewed to ensure that they are current and properly executed. Work is being done with the Calgary Lab on a method development for testing pesticide residues in organic fertilizers, like compost.Number of samples analyzed was 131 for pesticide guarantee verification and 63 for pesticide contamination audit. |
|
3. DFO
|
(d) Monitor and research presence and effects of pesticides in marine and freshwater ecosystems. |
$7.9 M |
$1.0 M |
$1.0 M |
Provide PMRA with final reports on four regional National Fund projects: 1) Impacts of forest spray programs on trout/salmon, Newfoundland-Labrador; 2) Effects of pesticides on fish reproduction, Quebec; 3) Impacts of pesticides on salmon habitat and on neurological development, Pacific; 4) Potential for biological effects from episodic release of pesticides into the aquatic environment, Gulf and Maritimes. Provide PMRA with a status report from DFO’s Centre for Environmental Research on Pesticides (CERP). CERP will conduct studies to quantify impacts of exposure to pesticide residues in two model systems in Canada: one representative of prairie land use and another indicative of southern Ontario land use pattern. Impacts will be quantified in terms of reproductive success of native fish populations as well as overall population numbers. After consultation with PMRA, DFO will design and initiate new research projects related to the theme -“Population Level Impacts of Pesticides on Fisheries Resources”. Contribute to Formative Evaluation of the Building Public Confidence in Pesticide Regulation and Improving Access to Pest Management Products Horizontal Initiative. |
DFO presented reports to PMRA at 5NR Workshop In Ottawa February 25-27, 2007. Summary written reports being assembled for submission to PMRA as a single document Comprises a portion of the summary report above. CERP conducted studies in Ontario and initiated collaboration with Dr. Annemieke Fairenhorst (University of Manitoba) to examine impacts in prairie wetlands. Four projects under this theme have been funded for 2007-2008. DFO contributed to development of a Memorandum of Understanding under which pesticide research results will be shared among the 5NR departments. Furthermore, DFO contributed to development of an integrated workplan for the 5NR departments. |
|
4. EC |
(e) Monitor and research presence and effects of pesticides in the environment. |
$7.0 M |
$1.0 M |
$1.0 M |
EC leads in specific research and monitoring themes have provided EC’s Pesticide Program Coordinating Committee with a document highlighting each theme’s results for the first three-year cycle of work. Themes include air and water surveillance; fish, amphibian and multitrophic aquatic effects; and plant, mammal and bird terrestrial effects. Following three years of research we will obtain answers to questions regarding knowledge generation with highlights of findings, contribution to the initial Pesticide |
Under Environment Canada's new management structure, the Pesticide Program falls under the Clean Water Result and is coordinated by the EC Pesticide Program Coordinating Committee. We have maintained and are continuing activities addressing the following:
|
|
4. EC cont’d |
|
|
|
|
Science Fund (PSF) objectives (e.g. national in scope and linked to regulatory decision-making priority, advanced knowledge of pesticide fate and effects), contribution to future departmental priorities, links within EC and to interdepartmental research/monitoring activities, leverage of complementary work and building of partnerships, scientific (or other) publications and finally, the theme’s top five priorities for PSF (including research, monitoring, methods development, risk assessment and modelling). These documents were used by the Committee to prioritize research and monitoring activities for a second cycle of work beginning in 2006-2007. Environmental priorities will be set according to the fundamentals of detecting change, understanding why it is occuring, better understanding what we can do about it, and using this information to inform decision makers and Canadians. Collected knowledge will be used in the context of EC’s Competitiveness and Environmental Sustainability Framework (CESF) and applied to pesticides. This will support decisions related to national competitiveness, protection of the health and safety of Canadians as well as to conservation of ecosystem functions. |
Continue to provide science advice to PMRA to meet regulatory data gaps, reduce knowledge deficiencies, and improve risk assessment methods. EC provided significant input into PMRA’s
Showcased PSF achievements at national and international venues to facilitate the exchange of information that will allow achievement of PSF objectives and use of this information to inform decision makers and Canadians.
Co-hosted 5NR Pesticide Research and Monitoring Workshop - EC presented on 11 topics. Hosted pesticide water monitoring workshops to present current data and better link with PMRA needs. Proceedings were produced. |
|
4. EC cont’d |
|
|
|
|
To better integrate and coordinate research with regulation, EC will continue to work with PMRA in implementation of the EC/PMRA MOU. The MOU has four components: Science Policy, Knowledge Generation, Issue Management and Compliance Promotion and Enforcement. EC will continue providing leadership in development and implementation of a coordinated federal pesticides science strategy for research and monitoring. As well, EC will continue to contribute to PMRA’s pesticide assessments where appropriate and will continue to provide science/policy advice on key Government of Canada policies as they relate to pesticide management and use. |
Met with industry to promote development of Canadian Environmental Quality Guidelines on pesticides. Establishing Canadian Council of Ministers of the Environment (CCME) sub-committee to focus solely on pesticide-related issues. Drafting 6NR MOU and workplans to establish mechanisms that facilitate exchange of scientific information and advice and promote strong working relationships among six federal partners. |
|
5. HC (PMRA) |
(f) Linking pesticide regulation and research. |
$4.2 M |
$0.8 M |
$0.8 M |
Identify PMRA’s research and monitoring priorities annually and communicate to 5NR partners through regular meetings and other avenues as needed. Facilitate discussion among the 5NR on identifying actions to address specific priorities, including collaborative research. Discuss with the 5NR how the results of their research and monitoring are used in regulatory decisions to build better linkages between research and regulation. Facilitate two-way communication and coordination between governments within Canada (through PMRA’s FPT Committee) and internationally as well as with the private and academic sectors, through presentations linking research and regulation at regional, national and international meetings e.g. through SETAC, CSA, IUPAC. To strengthen the framework in linking pesticide research and monitoring, develop a MOU amongst the 5NR. Improve risk assessment procedures particularly in environmental fate prediction e.g. water modelling and exposure assessment. Continue to improve and expand the use of probabilistic risk assessments. |
PMRA hosted a 6NR workshop that included provincial and industry representatives. The workshop provided a forum where pesticide regulators and researchers from the 5NR departments could exchange information on pesticide research and monitoring and how these fit into the regulatory framework; PMRA assessment and mitigation of pesticide risk to the environment and results of recent 5NR pesticide research in environmental toxicology and environmental fate, and environmental monitoring. There was discussion on future research and monitoring priorities, and linkages to the current PMRA re- evaluation schedule. Subsequently, a draft 6NR integrated workplan was completed and circulated to the 6NR departments for comments (to be finalized in spring 2007). A 6NR DG level committee was formed, an MOU was produced and circulated amongst the members for approval (spring 2007). A new NAFTA project on degradation kinetics was initiated. This will provide tools and guidance for both assessment and modelling purposes. A tiered approach to aquatic exposure assessment has been fully implemented. The Directorate is improving its capacity for using and assessing probabilistic risk assessment methods on several fronts. First, an introductory course on probabilistic risk assessment was delivered to the entire directorate in February 2007. In addition, four scientists within the Directorate have been chosen to form a probabilistic technical working group. This group will receive advanced training on probabilistic methods this fiscal year and will provide expert advice and/or analytical support on issues related to probabilistic risk assessments. A probabilistic approach has been used in two recent assessments. |
|
5. HC (PMRA) |
(g) Conducting research to support introduction of minor-use pesticides that pose a reduced risk to the environment. |
$3.5 M |
$0.9 M |
$0.9 M |
Advance risk assessment methodologies through: 1 - Further refinement and application of environmental protection goals; 2 - Publication of draft guidance on environmental risk assessment methods; 3 - Research to support harmonization of risk assessment methodology with international partners e.g. occupational exposure assessment, ground water modelling, pesticide degradation kinetics. Facilitate access to reduced-risk products, specifically low risk products, through developing, and publishing for external comment, guidance on registration of low risk products. Continue to develop a database on environmental toxicology and fate to guide decisions, internally and externally, on comparative risk and reduced-risk products. Finalize and publish a Best Management Practices guide to reduce spray drift by applicators. Publish for public comment a document identifying various options to better communicate buffer zones on labels to applicators. |
A 6NR integrated workplan is under development. A draft guidance on environmental risk assessment methods within the Directorate is being revised. To facilitate residue chemistry assessments, crop group schemes to incorporate additional Minor Use crops (NAFTA/CODEX) were developed or expanded. Also crop profiles were developed with AAFC for ornamentals. Additionally, Dietary Risk Assessments (DRA) were facilitated for Minor Use crops. Agricultural statistics were updated based on most recent consumption data for DRA and Maximum Residue Limits (MRL) setting that is undergoing validation. (HED) A new NAFTA project with the U.S. Environmental Protection Agency (EPA) on degradation kinetics was initiated. A NAFTA project with the U.S. EPA on ground water model selection, scenario and guidance development continues. Guidance document on low risk pesticides has been drafted and discussed with PMAC, FPT and numerous industry associations. Guidance document is in editing/translation. Development and transfer of database from Microsoft Access to the Agency Pesticide database (Oracle) was initiated. An MOU with AAFC for providing data for their National Agr-Environmental Health Analysis and Reporting Program (NAHARP) indicator project was developed and approved. Finalization and publication of the documents has been delayed due to submission workload |
|
6. NRCan |
(h) Research and monitor pesticides in the forest environment. |
$3.5 M |
$0.4 M
|
$0.4 M |
Third and final year of research work for four projects will be completed. Provide results to clients/stakeholders and PMRA in reports and publications. Research projects are: 1) Environmental fate and ecological effects of a systemic insecticide for control of exotic wood boring insect pests. Completion of second year field research program (GLP study comparing fate of imidacloprid following soil and stem injections); 2) Development of a biological treatment for control of root rot pathogen and impact on microbial biodiversity; 3) Advanced methods for monitoring impacts of pest control products on key microbial communities of forest soils. Publish guidelines that demonstrate the use of cutting edge molecular methods to study environmental fate of microbes; 4) Monitoring status of spruce budworm population to improve forest protection programs, integration of information on mortality agents in infestation forecasting and spray decision making for spruce budworm. Refine research priorities and plan for request for new proposals, January 2007. |
Final Reports and publications were completed and data submitted for the four projects, in support of product registration. Research presentations were made at a session entitled Enhanced Pest Management Perspective, Forest Pest Management Forum (December 2006). Participated in the 6NR Environmental Information Exchange on Pest Management Research and Monitoring in Canada 2007 (February 2007) hosted by PMRA. The main objective was to provide a forum for sharing of information and data. The following papers presented were by NRCan-CFS:
A new cycle of proposals is to be initiated in 2007-2008. |
|
AAFC HC (PMRA) |
II. DEVELOPING AND IMPLEMENTING COMMODITY SPECIFIC RISK REDUCTION STRATEGIES | |||||
|
1. AAFC |
(a) Commodity-based risk reduction strategies. |
$19.3 M |
$2.5 M |
$0.40 M SAL 0.07 M EBP 1.53 M NPO .45 M PWG --------- $2.45 M Total |
Process to engage stakeholders in crop prioritization based on risk and needs assessments developed. Next wave of about 10 crop profiles to be finalized and published. Develop up to five risk reduction strategies and support implementation of priority projects as established with Technical Working Group/stakeholders. Fund research and implementation projects from November 2005 Call for Proposals. Follow-up from workshop on barriers to grower adoption of IPM practices. Analyze data from pilot pesticide use survey. Continued implementation of AAFC/PMRA joint communication plan. |
More than 30 meetings held with stakeholders of priority commodities to determine key pest management issues for risk reduction and to develop strategies to deal with these issues. Twelve crop profiles published on website (4 new crop profiles and 8 updates completed). This brings to 26 the total number of crop profiles web published as of March 31, 2007. Strategy consultations undertaken for 19 crops. More than 30 pest management issues for risk reduction identified and 20 strategies developed. Strategy development commenced for 16 others. Oversight and funding provided for 34 ongoing projects. Five projects completed and final reports submitted in April 2006. An action plan for communication and technology transfer of the results is in progress. Call for Proposals launched in fall 2006 requiring establishment of new process with PWGSC was open to AAFC and external research/contractor community. Peer review of proposals completed, resulting in selection of 35 new projects for funding which address key pest management issues identified in pesticide risk reduction strategy consultations. Announcement regarding new projects planned for Q2, 2007-2008. The IPM Working Group wrapped up its activities at the end of March 2007. The group held four meetings to develop a national approach to support grower adoption of IPM practices and to develop guidelines for pilot projects. Recommendations of the group were presented to the PRR Program and its Technical Working Group and are being taken into consideration in the context of future directions of the Program. Delays due to the complexity of the data collected have slowed analysis of the crop protection survey. A draft publication has been prepared and will be completed early in 2007-2008. A new approach is being developed for analysis of IPM data in the questionnaire. Various communications products were released (fact sheets, reports, CDs, etc.). |
|
2. HC (PMRA) |
(a) Commodity-based risk reduction strategies (RR) |
$25.7 M |
$4.0 M (2.0 M for commodity strategies / 2.0 M for RR product review) |
$4.0 M |
Planned staffing actions of indeterminate positions. Ongoing consultations with stakeholders; work share with other government departments and 5NRs. Work on pesticide risk indicator: consult, build and validate database. Refine, together with AAFC, prioritization criteria for determining priority crops for the program. Work share with AAFC on crop profiles. Risk reduction strategies have been developed for pulse crops and canola. A long-term fireblight management strategy has been developed for apples. Steering committee and working groups have been meeting to develop solutions to identified priorities and implement steps to resolve these issues. Substantial progress has been made in developing strategies and forming steering committees to lead the strategies for a number of other crops: greenhouse vegetables, grape, peach, potato, strawberry and apple. Pursue risk reduction program for honey, Richardson ground squirrel and develop a work plan for forestry uses and needs. Consolidate and integrate all information collected with this program into the registration stream of PMRA. Continue review of reduced-risk pesticides submitted for registration. |
Two NAFTA microbial Joint Reviews completed. Pesticide Risk Indicator (CaPRI): 1- FPT consultation completed. 2- First report circulated within FPT and comments addressed. 3- Two documents being written are to be published in 2007. 4- Improvement of model under way for publication of second report which is expected to be made public , followed by consultations in 2008. 5- Three databases needed (human health data, environmental data, and pesticide use/sale data) are being completed to assess the pesticide risk trend of all provinces (as opposed to only Quebec and Ontario. (VSAD) Registered two new microbial products for control of fireblight disease on apples. Ongoing: six microbial, two pheromone and five low risk (biochemical) products. |
|
AAFC HC (PMRA) NRCan |
III. GENERATION OF DATA TO SUPPORT REGISTRATION OF REDUCED-RISK AND MINOR-USE PESTICIDES FOR THE AGRICULTURAL AND AGRI-FOOD SECTOR AND REDUCED-RISK PESTICIDES AND BIOPESTICIDES FOR FORESTRY | |||||
|
1. AAFC
|
(a) Improving access to agricultural minor-use pesticides, and reduced-risk pesticides for agricultural use. |
$33.7 M $12.0 M A-base |
$6.5 M $2.0 M A-base |
$1.94 M SAL 0.39 M EBP 3.04 M NPO 0.85 M PWG $6.25 M Total $2.0 M A-base |
Thirty-six pest-crop combinations will be identified at annual national stakeholder meeting hosted by AAFC. Manufacturer (registrant) written support will be obtained by July 2006 for each pest-crop pair, then sent to PMRA for review by October with the majority by August (PSCR 3.1). Subsequently, data requirements (DACO) for each pest-crop pair will be issued by PMRA to AAFC according to PMRA-established timeline (97 days from receipt). AAFC will convert DACOs to study plans by January 2007 and assign trials that complete the study plans, to contractors and collaborating AAFC personnel across Canada. Good laboratory practice (GLP) trials require quality assurance oversight that is provided by contractors and AAFC headquarters staff. |
[PMRA/HED] Participated in National Priority Setting meeting In addition to 38 priorities established during AAFC’s MU Priority Setting Workshop (March 26-28, 2007), we selected 25 joint AAFC/U.S. minor-use projects during IR-4's planning meeting (November 1-3, 2006). D3.1s (AAFC) 26 presubmissions and 11 sets of trial requirements were issued. (HED) Registrant written support was obtained and Pre-Submission Consultation Requests (PSCR) were submitted to PMRA for each priority by October 2006. DACO’s were not all received by January 2007. As a result this activity continued to year-end. It was determined that since AAFC is working with both registered and unregistered compounds, PMRA does not want AAFC to submit PSCRs for unregistered compounds. Study plans are being written and data collection is being initiated for 354 field trials. All residue trials respected GLP requirements without any significant observations. Also, in January 2007 a Standard Council of Canada GLP audit was conducted on our facility. There were no major observations, only minor clarifications. A response was provided to SCC by the required deadline. PMC accreditation to OECD GLP standards will continue. |
|
1 AAFC cont’d |
|
|
|
|
Data generation from field trials in 2006 and laboratory analysis of residues proceeds to final report stage in spring–summer 2007 and are submitted to PMRA. PMRA provides a decision on use 247 days later. Total process takes about 36 months. |
D3.2s (AAFC) 7 submissions (HED) More than 400 field trials from 2006 were completed and final reports are being written and assessed in order to be submitted to PMRA. Completed and submitted 41 AAFC projects or registrants for submission to PMRA. |
|
2. HC (PMRA) |
(a) Improving access to agricultural minor-use pesticides, and reduced-risk pesticides for agricultural use. |
$20.8 M |
$4.0 M |
$4.0 M |
Product evaluation work—review presubmission proposals from AAFC and provincial coordinators and issue data requirements. Register new minor crop uses, including minor-use and reduced-risk products and uses. Harmonization work and regulatory projects—Joint Reviews in collaboration with U.S. EPA, AAFC and U.S. Department of Agriculture IR-4 Program, further work on crop groupings and on Maximum Residue Levels (MRL) promulgation. Increase communication and provide feedback to AAFC, to improve quality and use of scientific rationales. |
Minor-Use Submissions: D3.1 - 92 presubmissions D3.2 - 28 submissions JR’s - 2 Minor-Use Registrations/MRLs: 28 label expansions 13 registrations resulted in MRL recommendations Meetings with AAFC regarding study report format, rationale preparations - ongoing Technical Gap Submissions - approximately two+ conventional chemicals, five microbial and six low risk (biochemical) new products registered. (HED) |
|
3. NRCan
|
(b) Develop and facilitate use of reduced-risk pesticides and biological pesticides for forestry. |
$4.1 M |
$0.3 M |
$0.3 M |
Review final reports of nine projects funded for three years and plan strategy and priorities for future funding. NRCan will continue work to integrate and coordinate activities with 5NR partners and stakeholders. The NRCan-CFS Minor-Use Advisor hired under this fund will continue to work in collaboration with AAFC to facilitate registration of reduced-risk/minor-use pest control products against pests on outdoor woody ornamentals and forests. Coordinate and report on six projects for minor-use pesticides in Canada. Support 2006 National Forest Pest Management Forum. Support forest projects on reduced-risk pest control products. |
Completed NRCan-CFS Enhanced Pest Management Methods Science & Technology Program Review 2002-2006 (March 2007). NRCan exchanged and shared information and data at 6NR departments’ Pesticides Committee meetings. Contributed to BPC Formative Evaluation Report (2006). NRCan-CFS Minor-Use Advisor housed at AAFC Pest Management Centre obtained support for minor-use products on two Christmas tree projects and a reforestation nursery that were useful for both forestry and agriculture. A group of three borers including emerald ash borer and Asian long-horned beetle were selected for screening of potential control products. A Data Package and Application for Registration for use of the insect growth regulator MIMIC against gypsy moth, were submitted to PMRA. A balsam fir sawfly nucleopolyhedrovirus, Abietiv™, received conditional registration from PMRA in April 2006, and was used operationally for control of the sawfly in Newfoundland. Provided financial and research support for 2006 National Forest Pest Management Forum which consists of pest managers, researchers, industry, regulators, and others interested in pest management. PMRA registered one new microbial pesticide and one new pheromone. |
|
TOTAL |
|
$154.96 M |
|
|
|
|
|
17. Results Achieved by Non-federal Partners: n/a |
||||||
|
18. Contact Information: |
19. Approved by:
|
20. Date Approved: |
||||
Table 17: Horizontal Initiative
| 1. Name of Horizontal Initiative: Federal Tobacco Control Strategy |
2. Name of Lead Department: Health Canada |
|||||
| 3. Start Date of the Horizontal Initiative: 2001-02 |
4. End Date of the Horizontal Initiative: 2006-07 and ongoing |
5. Total Federal Funding Allocation: $560 M (See note about Health Canada total funding in #11.) |
||||
|
6. Description of the Horizontal Initiative: The Federal Tobacco Control Strategy (FTCS) establishes a framework for a comprehensive, integrated, and multi-faceted approach to tobacco control. The FTCS is the federal contribution to the national tobacco control plan endorsed in 1999 by all Ministers of Health. It focuses on four mutually reinforcing components: protection, prevention, cessation and harm reduction, supplemented by effective use of public education campaigns to reach all Canadians. |
||||||
|
7. Shared Outcome: The FTCS has five 10-year objectives (2001-11):
|
||||||
|
8. Governance Structure: Resources for implementation were allocated to a number of departments and agencies. Health Canada (HC) is the lead department and is responsible for regulating the manufacture, sale, labelling and promotion of tobacco products as well as developing, implementing and promoting initiatives that reduce or prevent the negative health impacts associated with smoking. The partner departments and agencies are:
|
||||||
|
9. Federal Partners Involved in each Program |
10. Names of Programs |
11. Total Allocation 2001-02 to 2006-07 |
12. Forecasted Spending for 2006-07 |
13. Actual Spending in 2006-07 |
14. Planned Results for 2006-07 |
15. Achieved Results in 2006-07 |
|
1. HC |
FTCS |
$482.5 M (Note: this original allocation has been affected by several cuts since the FTCS began. |
$80.8 M (Tobacco Control Program: $67.8 M) |
$75.6 M (TCP - $66.6 M) |
See text below. |
See text below. |
|
2. PS |
FTCS |
$3.2 M |
$0.6 M |
$0.6 M |
See text below. |
See text below. |
|
3. ODPP |
FTCS |
$10 M |
$1.3 M |
$1.3 M |
See text below. |
See text below. |
|
4. RCMP |
FTCS |
$10.5 M (To offset seven analytical FTEs and five technical support FTEs) |
$1.5 M |
$1.5 M |
See text below. |
See text below. |
|
5. CRA |
FTCS |
$43.1 M (Total allotment to CRA, includes Customs/CBSA $30.7 M and Assessment and Benefit Services $1.7 M and Legislative Policy and Regulatory Affairs Branch $10.7 M) |
$10.4 M (Allocated between Customs/ CBSA and two CRA areas) |
$10.4 M (Allocated between Customs/ CBSA and two CRA areas) |
See text below. |
See text below. |
| Assessment and Client Services (previously Assessment and Collections) | See above. | See above. | $.2 M (Included in above) | See text below. | See text below | |
| Excise and GST/HST Rulings Directorate/ Legislative Policy and Regulatory Affairs Branch | See above. | $.5 M (Included in above) | See text below. | See text below. | ||
|
6. CBSA Intelligence Directorate and Travellers Division |
FTCS |
$21.1 M |
$5.1 M for activities plus $4.3 M for loss of duty-free licensing |
$5.1 M for activities plus $4.3 M for loss of duty-free licensing |
See text below. |
See text below. |
|
|
|
Total $560 M |
Total $104.0 M |
Total $98.8 M |
|
|
|
16. Comments on Variances: The Health Canada variance is mainly the result of a budget reduction sustained as part of the 2004 government-wide Expenditure Review exercise and a reallocation of FTCS resources to Health Canada's responsibilities with respect to the Canadian Environmental Protection Act. This reduction is permanent. As part of the September 2006 Expenditure Review, the FNIHB portion of the FTCS funding was eliminated. This reduced Health Canada's overall budget by $10.8 M over the next two years (reduction of $2.5 M in 2006-07; $8.3 M in 2007-08; $10.8 M in 2008-09 and ongoing). |
||||||
|
17. Results Achieved by Non-federal Partners: Through funding provided by the FTCS, the Akwesasne Mohawk Police (AMP) have been able to increase their surveillance and monitoring of tobacco smuggling. The AMP has reported participating in joint forces operations that have led to charges and seizures, including tobacco. All tobacco seizures made by the AMP are turned over to the RCMP for prosecutions and reported through the RCMP Cornwall Detachment. The AMP have enhanced their capacity in intelligence development and specialized criminal investigation techniques through their work with Canadian and U.S. law enforcement partners in the context of the Integrated Border Enforcement Team in the Cornwall area. In addition, they have had an opportunity to lead and participate in Joint Forces Operations related to cross-border criminal activities and organized crime. An evaluation is being conducted by Consulting and Audit Canada under contract to PS. The final evaluation report is anticipated by July 30, 2007. |
||||||
|
18. Contact Information: Dave Semel (613) 952-3367 |
19: Approved By: | 20. Date Approved: | ||||
| 14. Planned Results for 2006-07 | 15. Results Achieved in 2006-07 | |||||
| 1. Health Canada (HC) | Complete an evaluation to assess the impacts of the first five years of the FTCS. | A summative (impact-based) evaluation was conducted of the FTCS (2001-06) including all Tobacco Control Program components i.e. contribution-funded projects, regulatory interventions, research and policy development; First Nations and Inuit Health Branch (FNIHB); International Affairs Directorate (IAD); and federal partners. | ||||
| Work with eight regional offices and partner with several National Aboriginal Organizations across Canada for program delivery and development. | HC supported over 100 First Nations and Inuit projects at the national, regional and community level aimed at cessation and tobacco awareness with a particular emphasis on youth, young adults and pregnant women. | |||||
| 2. PS | Enhanced partnership arrangement with Akwesasne Mohawk Police. | See Results Achieved by Non-federal Partners above. | ||||
| 3. ODPP | 1) Prioritize fine recovery for fines ordered under cigarette contraband and tobacco sales to youth convictions. | 1) The number of cigarette contraband and other tobacco-related fines has gone from 1,920 files in 2002 to 892 files as of March 31, 2007, a reduction of over 53.5 percent. There were 826 files in the inventory in 2005-06. This year's figure is mostly due to the increase in convictions for selling tobacco to minors and an increase in cigarette contraband which has resulted in more convictions. | ||||
| 2) Increase the number of fines satisfied by a minimum of 15 percent. | 2) The number of fines that were satisfied as of March 2007 was 423 as compared to 491 in March 2006, a reduction of 14 percent. This may be due to the fact that additional emphasis is being given to attempting to recover long-standing fines which by nature are more difficult to recover. | |||||
| 3) Analyze trends and prioritize the most effective and least costly recovery methods. | 3) Priority is given to the most cost-effective methods of recovery, in particular, demand letters, telephone calls and negotiating payment agreements. To date, approximately 27,000 interventions have been made, resulting in the recovery of over $32 M in past-due fines as of March 2007. | |||||
| 4) Prioritize payment of fines over incarceration, but enhance enforcement measures when appropriate. | 4) Incarcerations for non-payment of fines totalled 66 in 2007 as compared to 60 in 2006. Of these 66 offenders, 15 subsequently opted to pay their fine rather than remain in incarceration. | |||||
| 5) Reduce costs to client departments in regards to fees incurred for Crown counsel attending motions for extensions in the delay to pay a fine. | 5) Crown counsel assigned to Fine Recovery Units oppose all motions for payment extensions heard at court, resulting in a decrease in counsel fees to client departments for these hearings. | |||||
| 4. RCMP | 1) Provide the Department of Finance, Health Canada and other partners with current updates on illicit tobacco trade activities. | 1) Regular reports on the illicit tobacco situation were provided to Finance and Health Canada. Side bar reports provided to other partners and key Ministerial entities upon request. Tobacco analysts attend regular meetings to brief the Department of Finance. | ||||
| 2) The RCMP monitors illegal activities at and along the Canada/U.S. border through the use of strategic detection and surveillance equipment. | 2) Improved border security through the use of sophisticated technology which permits detection and monitoring of illegal border intrusions, resulting in vital intelligence. | |||||
| 3) Expand cooperation with international and national law enforcement partners. | 3) Co-hosted the 2006 Joint U.S./Canada Tobacco Diversion Workshop with American and Canadian agencies. The RCMP holds biannual Joint Smuggling Initiative conferences across the country to address current trends and ongoing investigations into the illegal tobacco trade. Partners are invited to share information and to build key partnerships to enhance investigations. | |||||
| 5. CRA | 1) Systems adjustments and maintenance to reflect the legislative changes that affect rates, reporting and refunds as well as program changes to include duty-free shops and ships stores. | 1) Systems maintained as required. Reporting capabilities were reviewed and enhanced to meet program requirements. | ||||
| Assessment and Benefit Services (previously Assessment and Collections) | 2) Verify export activity. | 2) The Tobacco Enforcement Verification Program (field) effectively monitored the movement of exported tobacco products. | ||||
| Excise and GST/HST Rulings Directorate/ Legislative Policy and Regulatory Affairs Branch | 3) Ensure legislative compliance with remittance requirements as well as stamping and marking. | 3) Excise duty officers increased the number of compliance and audit visits to licensed manufacturers to ensure compliance with remittance requirements as well as stamping and marking. | ||||
| 4) Work with stakeholders to monitor and assess the effectiveness of measures used to reduce contraband tobacco. | 4) Participated on a number of committees dealing with the monitoring and control of tobacco products, including those dealing with interprovincial issues. Co-hosted the 2006 Tobacco Diversion Workshop with Canadian and U.S. participation. | |||||
| 5) Provide the Department of Finance with advice to assist in the determination of the magnitude and timing of future tax increases. | 5) Met with the Department of Finance as required. Provided industry and product information. | |||||
| 6) Support RCMP enforcement activity. | 6) Supported RCMP enforcement activity by providing information about specific tobacco transactions as well as expert testimony and affidavits. | |||||
| 6. CBSA Intelligence Directorate |
1) Provide advice to Department of Finance on matters that will impact the future tax structure on tobacco. | 1) Attended monthly meetings with Department of Finance and partners to discuss and serve as a reference for questions on tobacco issues. | ||||
| 2) Monitor and report on the contraband tobacco situation in Canada. | 2) Provided monthly analysis of the national contraband situation by compiling reports received from the Regions. Partnered with RCMP in annual risk assessment of the nature and extent of tobacco contraband activity. Coordinated development of tobacco intelligence in the Regions. The capabilities of our officers/analysts to infiltrate the marketplace, gather intelligence, liaise with other agencies and process their files has resulted in: an increase in targets for examination, both companies and individuals; identification of possible risk elements not previously perceived; awareness of emerging trends and threats. | |||||
| 3) Expand cooperation with international and national law enforcement partners. | 3) Participated in Joint Force Operations with law enforcement partners across the Regions. Co-hosted the 2006 Tobacco Diversion Workshop with American and Canadian agencies. Developed and maintained contact with international tobacco enforcement personnel. | |||||
| Travellers Division | Collection of the tobacco duties imposed on personal importations of returning Canadians. | CBSA front-line officers collected duties and taxes from previously exempted personal importations of tobacco. | ||||
Table 19: Storage Tanks
In order to reduce the volume of printed material, this table is not to be included in the printed DPR.
|
Status of Fuel Storage Tanks on Health Canada crown-owned Land |
|
|
As required under the Canadian Environmental Protection Act (CEPA), Part IV, Federal Registration of Storage Tank Systems for Petroleum Products and Allied Petroleum Products on Federal Lands or Aboriginal Lands Regulations, this report provides the information set out in Schedule II of the aforementioned regulations, updated to December 31,2006. |
|
|
The following number of aboveground storage tank systems: |
|
|
Are registered with Health Canada |
6 |
|
Comply with the Federal Aboveground Storage Tank Technical Guidelines |
4 |
|
Do not comply with the Federal Aboveground Storage Tank Technical Guidelines |
2* |
|
The following number of underground storage tank systems: |
|
|
Are registered with Health Canada |
5 |
|
Comply with the Federal Underground Storage Tank Technical Guidelines |
2 |
|
Do not comply with the Federal Underground Storage Tank Technical Guidelines |
3** |
*One of the non-compliant aboveground storage tanks has been removed since December 31, 2006, and Health Canada is reviewing the possibility of decommissioning the second aboveground storage tank.
**The removal of 3 non-compliant underground storage tanks (UST) is being reviewed by Health Canada and removal will be planned over the next 3 fiscal years (07/08 - 09/10).