Common menu bar links
Breadcrumb Trail
ARCHIVED - Patented Medicine Prices Review Board Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
SECTION III - SUPPLEMENTARY INFORMATION
Organizational Information
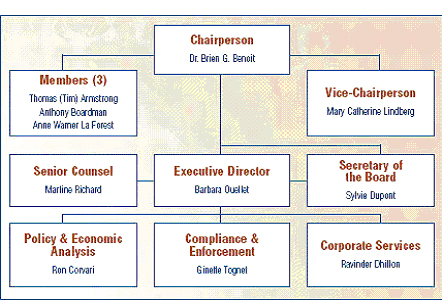
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and Vice-Chairperson. The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. The Executive Director manages the work of the staff. Senior staff consists of the Executive Director, the Director of Compliance and Enforcement, the Director of Policy and Economic Analysis, the Director of Corporate Services, the Secretary of the Board and Senior Counsel.
The Compliance and Enforcement Branch is largely responsible for the review of prices of patented medicines and the Compliance and Enforcement Policy. The Policy and Economic Analysis Branch is largely responsible for conducting analyses and preparing reports on price trends and other policy and economic studies. The Secretariat, Corporate Services Branch and Senior Counsel provide regulatory, reporting and administrative support.
Financial Table 1: Comparison of Planned to Actual Spending (including FTEs)
| ($ thousands) | 2004-05 Actual | 2005-06 Actual | 2006-2007 | |||
| Main Estimates | Planned Spending | Total Authorities | Total Actuals | |||
| Patented Medicine Prices Review Board | 4,996.7 | 5,326.5 | 6,512.0 | 6,512.0 | 11,690.0 | 7,365.3 |
| Total | 4,996.7 | 5,326.5 | 6,512.0 | 6,512.0 | 11,690.0 | 7,365.3 |
| Less: Non Respendable revenue(1) | (3,026.1) | (1,413.3) | - | - | - | (210.0) |
| Plus: Cost of services received without charge | 825.9 | 791.6 | 871.2 | 871.2 | 871.2 | 807.9 |
| Total Departmental Spending | 2,796.5 | 4,704.8 | 7,383.2 | 7,383.2 | 12,561.2 | 7,963.2 |
|
|
||||||
| Full Time Equivalents | 42 | 42 | 48 | 48 | 63 | 43 |
(1) The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province respecting the distribution to that province of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
Financial Table 2: Resources by Program Activity
| 2006-2007 | ||||||
| Program Activity | Budgetary | Plus: Non-Budgetary | Total | |||
| Operating | Total: Gross Budgetary Expenditures | Less: Respendable Revenue | Total: Net Budgetary Expenditures | Loans, Investments and Advances | ||
| Patented Medicine Prices Review Board | ||||||
| Main Estimates | 6,512.0 | 6,512.0 | - | 6,512.0 | - | 6,512.0 |
| Planned Spending | 6,512.0 | 6,512.0 | - | 6,512.0 | - | 6,512.0 |
| Total Authorities | 11,690.0 | 11,690.0 | - | 11,690.0 | - | 11,690.0 |
| Actual Spending | 7,365.3 | 7,365.3 | - | 7,365.3 | - | 7,365.3 |
Financial Table 3: Voted and Statutory Items
| Vote or Statutory Item | Truncated Vote or Statutory Wording | 2006-2007 | |||
| Main Estimates | Planned Spending | Total Authorities | Actual | ||
| 25 | Operating expenditures | 5,800.0 | 5,800.0 | 10,978.0 | 6,742.5 |
| (S) | Contributions to employee benefit plans | 712.0 | 712.0 | 712.0 | 622.8 |
| Total | 6512.0 | 6,512.0 | 11,690.0 | 7,365.3 | |
Financial Table 4: Services Received Without Charge
| ($ millions) | 2006-2007 Total Actual Spending |
| Accommodation provided by Public Works and Government Services Canada | 489.9 |
| Contributions covering employers' share of employees' insurance premiums and expenditures paid by the Treasury Board of Canada Secretariat (excluding revolving funds) | 299.7 |
| Salary and associated expenditures of legal services provided by the Department of Justice Canada | 18.3 |
| Total 2006-2007 Services received without charge | 807.9 |
(1) The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings or Board orders to offset excess revenues. The Minister may enter into agreements with any province respecting the distribution to that province of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
Financial Table 5: Sources of Non-respendable Revenue
| ($ millions) | Actual 2004-05 | Actual 2005-06 | 2006-2007 | |||
| Main Estimates | Planned Revenue | Total Authorities | Actual | |||
| Patented Medicine Prices Review Board | ||||||
| Source of non-respendable revenue(1) | ||||||
| Voluntary Compliance Undertakings | 3,026.1 | 1,413.3 | - | - | - | 210.0 |
| Total Non-Respendable Revenue | 3,026.1 | 1,413.3 | - | - | - | 210.0 |
(1) The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues. The Minister may enter into agreements with any province respecting the distribution to that province of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts.
Financial Table 6: Financial Statements of the Patented Medicine Prices Review Board
Statement of Management Responsibililty
Responsibility for the integrity and objectivity of the accompanying financial statements for the year ended March 31, 2007 and all information contained in these statements rests with management. These financial statements have been prepared by management in accordance with Treasury Board accounting policies which are consistent with Canadian generally accepted accounting principles for the public sector.
Management is responsible for the integrity and objectivity of the information in these financial statements. Some of the information in the financial statements is based on management's best estimates and judgment and gives due consideration to materiality. To fulfil its accounting and reporting responsibilities, management maintains a set of accounts that provides a centralized record of the Board's financial transactions. Financial information submitted to the Public Accounts of Canada and included in the Board's Departmental Performance Report is consistent with these financial statements.
Management maintains a system of financial management and internal control designed to provide reasonable assurance that financial information is reliable, that assets are safeguarded and that transactions are in accordance with the Financial Administration Act , are executed in accordance with prescribed regulations, within Parliamentary authorities, and are properly recorded to maintain accountability of Government funds. Management also seeks to ensure the objectivity and integrity of data in its financial statements by careful selection, training and development of qualified staff, by organizational arrangements that provide appropriate divisions of responsibility, and by communication programs aimed at ensuring that regulations, policies, standards and managerial authorities are understood throughout the Board.
The financial statements of the Board have not been audited.
 |
|
| Brien G. Benoit, M.D. Chairperson Patented Medicine Prices Review Board September 6, 2007 |
Barbara Ouellet Executive Director & Senior Financial Officer Patented Medicine Prices Review Board August 30, 2007 |
Statement of Operations (unaudited)
| for the year ended March 31 | 2007 | 2006 |
| (in dollars) | ||
| Expenses | ||
| Salaries and employee benefits | 4,815,847 | 4,084,405 |
| Professional and special services | 1,951,204 | 871,998 |
| Accommodation | 489,894 | 582,600 |
| Utilities, material and supplies | 484,531 | 247,009 |
| Travel and relocation | 181,186 | 85,756 |
| Purchased repair and maintenance | 124,330 | 70,340 |
| Information | 122,086 | 55,496 |
| Communication | 83,510 | 76,516 |
| Rentals | 16,014 | 10,133 |
| Amortization | 3,101 | 3,383 |
| Other | 55,634 | 118,942 |
| 8,327,337 | 6,206,578 | |
| Revenues | ||
| Voluntary compliance undertakings | 210,043 | 1,413,415 |
| Net cost of operations | 8,117,294 | 4,793,163 |
| The accompanying notes form an integral part of the financial statements | ||
Statement of Financial Position (unaudited)
| As at March 31 | 2007 | 2006 |
| (in dollars) | ||
| Assets | ||
| Financial assets Accounts receivable and advances (Note 4) |
108,595 |
35,819 |
| 108,595 | 35,819 | |
| Non-financial assets Tangible capital assets (Note 5) |
3,101 |
6,484 |
| - | 3,101 | |
| 108,595 | 38,920 | |
| Liabilities and Equity of Canada | ||
| Liabilities Accounts payable and accrued liabilities Vacation pay and compensatory leave (Note 6) Employee severance benefits (Note 7) |
784,600 266,437 733,660 |
453,184 200,419 645,076 |
| 1,784,697 | 1,298,679 | |
| Equity of Canada | (1,676,102) | (1,259,759) |
| 108,595 | 38,920 | |
| The accompanying notes form an integral part of the financial statements | ||
Statement of Equity (unaudited)
| As at March 31 | 2007 | 2006 |
| (in dollars) | ||
| Equity of Canada, beginning of year | (1,259,759) | (680,523) |
| Net cost of operations | (8,117,294) | (4,793,163) |
| Current year appropriations used (Note 3) | 7,365,303 | 5,326,472 |
| Revenues not available for spending | (218,605) | (1,413,557) |
| Change in net position in the Consolidated Revenue Fund (Note 3) | (253,685) | (490,541) |
| Services received without charge by other government departments (Note 8) | 807,938 | 791,553 |
| Equity of Canada, end of year | (1,676,102) | (1,259,759) |
| The accompanying notes form an integral part of the financial statements | ||
Statement of Cash Flow (unaudited)
| For the year ended March 31 | 2007 | 2006 |
| (in dollars) | ||
| Operating activities | ||
| Net cost of operations | 8,117,294 | 4,793,163 |
| Non-cash items: Amortization of capital assets (Note 5) Services provided without charge by other government departments (Note 8) |
(3,101) (807,938) |
(3,383) (791,553) |
| Variations in Statement of Financial Position: Increase (decrease) in accounts receivable and advances Decrease (increase) in liabilities |
72,776 (486,018) |
(589,360) 13,507 |
| Cash used for operating activities | 6,893,013 | 3,422,374 |
| Financing activities | ||
| Net cash provided by Government of Canada | (6,893,013) | (3,422,374) |
| The accompanying notes form an integral part of the financial statements | ||
Notes to the Financial Statements (unaudited)
1. Authority and purpose
The Patented Medicine Prices Review Board (PMPRB) is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (Act).
Although the PMPRB is part of the Health Portfolio, it carries out its mandate at arms-length from the Minister of Health. It also operates independently of other bodies such as Health Canada, which approves drugs for safety and efficacy, and public drug plans, which approve the listing of drugs on their respective formularies for reimbursement purposes.
The PMPRB has a dual role:
- Regulatory: To ensure that prices charged by patentees for patented medicines sold in Canada are not excessive thereby protecting consumers and contributing to Canadian health care;
- Reporting: To report on pharmaceutical trends and on the R&D spending by pharmaceutical patentees, thereby contributing to informed decisions and policy making.
Jurisdiction
Regulatory - The PMPRB is responsible for regulating the prices that patentees charge, the "factory-gate" price, for prescription and non-prescription patented drugs sold in Canada, to wholesalers, hospitals or pharmacies, for human and veterinary use to ensure that they are not excessive. The PMPRB regulates the price of each patented drug product, including each strength of each dosage form of each patented medicine sold in Canada. This is normally the level at which Health Canada assigns a Drug Identification Number (DIN).
The PMPRB has no authority to regulate the prices of non-patented drugs, including generic drugs sold under compulsory licenses, and does not have jurisdiction over prices charged by wholesalers or retailers nor over pharmacists' professional fees. Also, matters such as distribution and prescribing are outside the purview of the PMPRB.
Reporting - The PMPRB reports annually to Parliament through the Minister of Health. The Annual Report, which covers each calendar year, includes a review of the PMPRB�s major activities, analyses of the prices of patented medicines and of the price trends of all drugs, and reports on the R&D expenditures as reported by pharmaceutical patentees.
2. Significant accounting policies
The financial statements have been prepared in accordance with Treasury Board accounting policies which are consistent with Canadian generally accepted accounting principles for the public sector.
Significant accounting policies are as follows:
(a) Parliamentary appropriations
The Board is financed by the Government of Canada through Parliamentary appropriations. Appropriations provided to the Board do not parallel financial reporting according to generally accepted accounting principles since appropriations are primarily based on cash flow requirements. Consequently, items recognized in the statement of operations and the statement of financial position are not necessarily the same as those provided through appropriations from Parliament. Note 3 provides a high-level reconciliation between the bases of reporting.
(b) Net Cash Provided by Government
The Board operates within the Consolidated Revenue Fund (CRF), which is administered by the Receiver General for Canada. All cash received by the Board is deposited to the CRF and all cash disbursements made by the Board are paid from the CRF. The net cash provided by Government is the difference between all cash receipts and all cash disbursements including transactions between departments of the federal government.
(c) Change in net position in the Consolidated Revenue Fund
The change in net position in the Consolidated Revenue Fund is the difference between the net cash provided by Government and appropriations used in a year, excluding the amount of non respendable revenue recorded by the Board. It results from timing differences between when a transaction affects appropriations and when it is processed through the CRF (See note 3(c) for a reconciliation between net cash provided by Government and current year appropriations used).
(d) Revenues
Revenues are accounted for in the period in which the underlying transaction or event occurred that gave rise to the revenues. Patented Medicine Prices Review Board revenues represent monies collected as a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board orders to offset excess revenues.
(e) Expenses
Expenses are recorded on an accrual basis:
- Vacation pay and compensatory leave are expensed as the benefits accrue to employees under their respective terms of employment.
- Services provided without charge by other government departments for accommodation and the employer's contribution to the health and dental insurance plans are recorded as operating expenses at their estimated cost.
(f) Employee future benefits
- Pension benefits: Eligible employees participate in the Public Service Pension Plan, a multiemployer plan administered by the Government of Canada. The Board's contributions to the Plan are charged to expenses in the year incurred and represent the total obligation to the Plan by the Board. Current legislation does not require the Board to make contributions for any actuarial deficiencies of the Plan.
- Severance benefits: Employees are entitled to severance benefits under labour contracts or conditions of employment. These benefits are accrued as employees render the services necessary to earn them. The obligation relating to the benefits earned by employees is calculated using information derived from the results of the actuarially determined liability for employee severance benefits for the Government as a whole.
(g) Accounts receivable
Accounts receivable are stated at amounts expected to be ultimately realized. They are mainly comprised of amounts to be recovered from other government Departments and the recovery is considered certain. As a result, no provision has been recorded as an offset against these amounts.
(h) Tangible Capital Assets
All tangible capital assets having an initial cost of $10,000 or more are recorded at their acquisition cost. The Board does not capitalize intangibles, works of art and historical treasures that have cultural, aesthetic or historical value, assets located on Indian Reserves and museum collections.
Amortization of capital assets is done on a straight-line basis over the estimated useful life of the asset as follows:
| Asset Class | Sub-asset Class | Amortization Period |
| Machinery and equipment | Computer equipment | 3-5 years |
(i) Measurement uncertainty
The preparation of these financial statements in accordance with Treasury Board accounting policies which are consistent with Canadian generally accepted accounting principles for the public sector requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses reported in the financial statements. At the time of preparation of these statements, management believes the estimates and assumptions to be reasonable. The most significant items where estimates are used are the liability for employee severance benefits and the useful life of tangible capital assets. Actual results could significantly differ from those estimated. Management's estimates are reviewed periodically and, as adjustments become necessary, they are recorded in the financial statements in the year they become known.
3. Parliamentary Appropriations
The Board receives most of its funding through annual Parliamentary appropriations. Items recognized in the statement of operations and the statement of financial position in one year may be funded through Parliamentary appropriations in prior, current or future years. Accordingly, the Board has different net cost of operations for the year on a government funding basis than on an accrual accounting basis. The differences are reconciled in the following tables:
| (a) Reconciliation of net cost of operations to current year appropriations used: | 2007 | 2006 |
| (in dollars) | ||
| Net cost of operations | 8,117,294 | 4,793,163 |
| Adjustments for items affecting net cost of operations but not affecting appropriations: | ||
| Add (Less): | ||
| Revenues not available for spending | 218,605 | 1,413,557 |
| Services provided without charge by other government departments | (807,938) | (791,553) |
| Amortization | (3,101) | (3,383) |
| Justice Canada legal fees | (4,979) | (7,595) |
| Allowance for vacation pay accrual | (54,429) | (1,370) |
| Allowance for time-off in lieu accrual | (11,589) | 2,315 |
| Allowance for severance benefits | (88,586) | (78,669) |
| Other expenses not charged to Appropriations | 26 | 7 |
| (751,991) | 533,309 | |
| Current year appropriations used | 7,365,303 | 5,326,472 |
| (b) Appropriations provided and used: | 2007 | 2006 |
| (in dollars) | ||
| Operating expenditures - Vote 25 | 10,978,025 | 5,081,000 |
| Statutory Amounts | 622,760 | 543,344 |
| Less: | ||
| Lapsed appropriations | (4,235,482) | (297,872) |
| Current year appropriations used | 7,365,303 | 5,326,472 |
| (c) Reconciliation of net cash provided by Government to current year appropriations used | 2007 | 2006 |
| (in dollars) | ||
| Net cash provided by Government | 6,893,013 | 3,422,374 |
| Revenue not available for spending | 218,605 | 1,413,557 |
| Change in net position in the Consolidated Revenue Fund Variation in accounts receivable and advances Variation in accounts payable and accrued liabilities Other Adjustments |
(72,776) 331,414 (4,953) |
589,361 (91,232) (7,588) |
| 253,685 | 490,541 | |
| Current year appropriations used | 7,365,303 | 5,326,472 |
4. Accounts receivable and advances
| 2007 | 2006 | |
| (in dollars) | ||
| Receivables from other Federal Government departments and agencies | 108,095 | 35,319 |
| Employee advances | 500 | 500 |
| 108,595 | 35,819 | |
5. Tangible capital assets
| Cost (in dollars) |
Opening Balance | Acquisitions | Disposals and write-offs | Closing balance |
| Machinery and equipment | 91,242 | - | - | 91,242 |
| 91,242 | - | - | 91,242 | |
| Accumulated Amortization (in dollars) |
Opening Balance | Amortization | Disposals and write-offs | Closing balance |
| Machinery and equipment | 88,141 | 3,101 | - | 91,242 |
| 88,141 | 3,101 | - | 91,242 | |
| Net book value | 3,101 | - | - | - |
| Amortization expense for the year ended March 31, 2007 is $3,101 (2006 - $3,383) | ||||
6. Vacation pay and compensatory leave
| (in dollars) | 2007 | 2006 |
| Allowance for vacation | 253,100 | 198,671 |
| Allowance for compensatory leave | 13,337 | 1,748 |
| 266,437 | 200,419 |
7. Employee benefits
(a) Pension benefits
The Board's employees participate in the Public Service Pension Plan, which is sponsored and administered by the Government of Canada. Pension benefits accrue up to a maximum period of 35 years at a rate of 2 percent per year of pensionable service, times the average of the best five consecutive years of earnings. The benefits are integrated with Canada/Qu�bec Pension Plans benefits and they are indexed to inflation.
Both the employees and the Board contribute to the cost of the Plan. The current and previous year expenses, which represent approximately 2.2 times (2.6 in 2005-06) the contributions by employees, amount to:
| (in dollars) | 2007 | 2006 |
| Expense for the year | 458,955 | 402,069 |
| 458,955 | 402,069 |
The Board's responsibility with regard to the Plan is limited to its contributions. Actuarial surpluses or deficiencies are recognized in the financial statements of the Government of Canada, as the Plan's sponsor.
(b) Severance benefits
The Board provides severance benefits to its employees based on eligibility, years of service and final salary. These severance benefits are not pre-funded. Benefits will be paid from future appropriations. Information about the severance benefits, measured as at March 31, is as follows:
| (in dollars) | 2007 | 2006 |
| Accrued benefit obligation, beginning of year | 645,076 | 566,407 |
| Expense for the year | 147,991 | 150,455 |
| Benefits paid during the year | (59,407) | (71,786) |
| Accrued benefit obligation, end of year | 733,660 | 645,076 |
8. Related party transactions
The Board is related as a result of common ownership to all Government of Canada departments, agencies, and Crown corporations. The Board enters into transactions with these entities in the normal course of business and on normal trade terms. Also, during the year, the Board received services which were obtained without charge from other Government departments as presented in part (a).
(a) Services provided without charge
During the year the Board received without charge from other departments. These services without charge have been recognized in the Board's Statement of Operations as follows:
| (in dollars) | 2007 | 2006 |
| Accommodation | 489,894 | 582,600 |
| Employer's contribution to the health and dental insurance plans | 299,709 | 197,000 |
| Justice Canada | 18,335 | 11,953 |
| 807,938 | 791,553 |
The Government has structured some of its administrative activities for efficiency and cost-effectiveness purposes so that one department performs these on behalf of all without charge. The costs of these services, which include payroll and cheque issuance services provided by Public Works and Government Services Canada and audit services provided by the Office of the Auditor General , are not included as an expense in the Board's Statement of Operations.
(b) Payables outstanding at year-end with related parties:
| (in dollars) | 2007 | 2007 |
| Accounts payable to other government departments and agencies | 32,043 | 36,304 |
1 As a result of the decision of the Treasury Board Meeting of June 22, 2006, the PMPRB received funding through Supplementary Estimates (A) of $4,914,625 to conduct public hearings and to modernize the Excessive Price Guidelines.
2 As a result of the June 2006 Treasury Board Meeting, the PMPRB received the additional funding referred to in note 1 through Supplementary Estimates A. Provisions had been approved by Treasury Board for the PMPRB to access Vote 5 should it be unable to cash manage through to December 2006. In order to mitigate the need to access Vote 5, some activities related to Guidelines modernization were delayed (e.g. consultation meetings with stakeholders were deferred to November). In addition, while the total projected number of hearings for 2006-2007 did materialize by the end of the fiscal year, the timing of the issuance of the Notices of Hearing was later than expected. Finally, given the time delay in staffing of new approved positions, work in other areas (i.e. analytical studies on pharmaceutical trends) was delayed as staff were assigned to meet more pressing demands. Therefore, as a result of all of the above, actual spending was less than approved authorities.
3 A registration number (drug identification number) that the Health Products and Food Branch of Health Canada assigns to each prescription and non-prescription drug product marketed under the Food and Drug Regulations. The DIN is assigned using information in the following areas: manufacturer of the product; active ingredient(s); strength of active ingredient(s); pharmaceutical dosage form; brand/trade name; and route of administration.
4 The Guidelines are published in the PMPRB's Compendium of Guidelines, Policies and Procedures, which is available on the Web site: www.pmprb-cepmb.gc.ca, under Legislation, Regulations, Guidelines.
5 Details of pricing information that patentees must file are contained in Section 4 of the Regulations. The Patentees' Guide to Reporting outlines the four classes of customer: hospital, pharmacy, wholesaler and other.
6 For the purposes of the Board's price review, a new patented drug product in 2006 is defined as any patented drug product introduced in Canada, or previously marketed but first patented between December 1, 2005, and November 30, 2006. The same approach is used for all years due to the timing of the filing requirements under the Patented Medicines Regulations, 1994.
7 For the purpose of this report, existing medicines include all patented drug products that were introduced prior to December 1, 2005.
8 The Hearing in the matter of Janssen-Ortho Inc. and the medicine Risperdal Consta was concluded by the acceptance of a VCU. For additional information see page 24 of this report.
9 The Hearing in the matter of 3M Canada Company and the medicine Airomir was concluded by the acceptance of a VCU. For additional information see page 23 of this report.
10 The full text of each VCU is available on the Web site: www.pmprb-cepmb.gc.ca, under Regulatory, Voluntary Compliance Undertakings.
11 The total number of hearings was subsequently reduced to eight as a result of the Hearing Panel's acceptance of a VCU to resolve the matter of 3M Canada and the medicine Airomir, and the matter of Janssen-Ortho and the medicine Risperdal Consta. On July 25, 2007, a further Notice of Hearing was issued in the matter of Abbott Laboratories Limited and the price of the medicine Zemplar bring the number of ongoing hearings to nine.
12 Details of this case are provided under Voluntary Compliance Undertakings on page 23.
13 These groups have been combined for reasons of confidentiality.
14 The Patented Medicines Regulations, 1994, require patentees to file information according to four customer classes; these classes which are hospitals, pharmacies, wholesalers and other are specified in the Patentees' Guide to Reporting developed by the Board.
15 Results for the fourth customer class, "Other", are not provided. Primarily, it is made up of healthcare institutions other than hospitals, such as clinics and nursing homes, and may include physicians, etc.
16 IMS Health's Retail Drug Monitor, 2006 (www.imshealth.com). IMS Retail Drug Monitor provides estimates of direct (i.e., from the manufacturing company) and indirect (i.e., through a wholesaler) drug purchases by pharmacies in 13 major markets: Argentina, Australia, Brazil, Canada, France, Germany, Italy, Japan, Mexico, New Zealand, Spain, the U.K. and the U.S. These figures are at ex-manufacturer prices and include all prescription and certain over-the-counter data.
17 See the Regulatory Impact Assessment Statement (RIAS) of the Patented Medicines Regulations, 1988, published in the Canada Gazette Part II, Vol. 122. No. 20 - SOR/DORS/88-474
