Common menu bar links
Breadcrumb Trail
ARCHIVED - Hazardous Materials Information Review Commission Canada
 This page has been archived.
This page has been archived.
Archived Content
Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after the date of archiving. Web pages that are archived on the Web are not subject to the Government of Canada Web Standards. As per the Communications Policy of the Government of Canada, you can request alternate formats on the "Contact Us" page.
Section II Analysis of Performance by Strategic Outcome
Strategic Outcome
Trade secret exemptions within WHMIS that balance the right of industry to withhold bona fide confidential business information with the right of employers and workers to be provided with complete and accurate information on the health and safety hazards posed by workplace chemicals.
Program activity: Claims exemption process
Financial Resources ($ thousands)
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
| 3,512 | 3,601 | 3,401 |
Human Resources
| Planned | Actual | Difference |
|---|---|---|
| 35 | 30 | 5(1) |
(1) The variance is due to HMIRC's challenge to meet full capacity.
Under this activity, HMIRC registers claims for exemption received from a supplier or employer who wishes to withhold critical proprietary information, decides on the validity of the claim, adjudicates and issues decisions on the compliance of material safety data sheet or label to which the claim relates, and administers an appeal process to these decisions.
Expected results
- The protection of valid confidential business information about suppliers' and employers' hazardous products.
- A mechanism for workers to be informed about the health and safety hazards of exposure to chemicals found in products associated with claims for exemption.
- A system that resolves disputes in a fair, efficient and cost-effective manner.
Key program: Claims processing
Financial Resources ($ thousands)
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
| 3,336 | 3,421 | 3,370 |
Under this activity, HMIRC registers claims, thereby enabling a company to sell and/or distribute its product while the claim is being processed. Then the validity of the claim for exemption is determined, based on the Hazardous Materials Information Review Regulations criteria, and the material safety data sheet is evaluated to ensure compliance with WHMIS requirements. Decisions are issued and published in the Canada Gazette.
| Expected Results | Indicators | |
|---|---|---|
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Performance Analysis
Manage the Workload
Claims Registration
In 2006–2007, the Commission registered 387 claims for exemption, which was virtually unchanged from the 388 claims registered during 2005–2006. Of that total, 97%, or 377 claims, were received with all the necessary information and were verified and registered within the seven-day turnaround time specified in the Commission's service standard.
The Commission exceeded the seven-day turnaround time for the remaining 3% because the claimants had to submit additional information to substantiate their claims before they could be verified and registered.
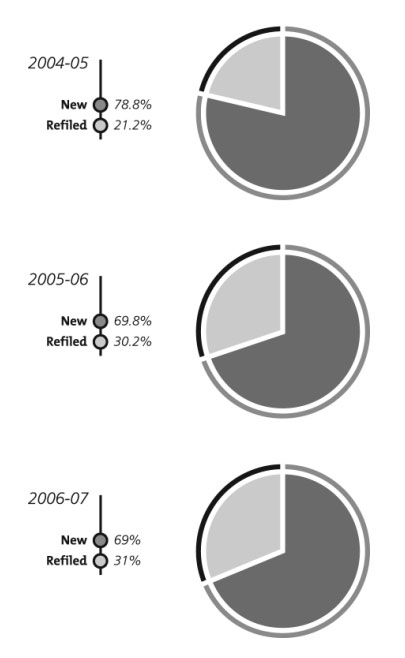
Consistent with the previous fiscal year, about 70% of the claims registered in 2006–2007 were new filings and 30% were previously approved claims that were refiled after three years, as the law requires (Figure 1).
Figure 1: Percentages of new and refiled claims registered, 2004–2005 to 2006–2007
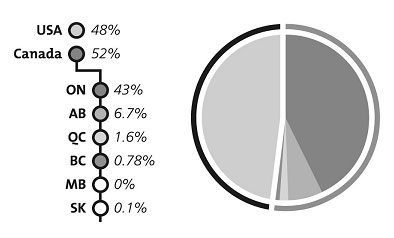
Consistent with previous years, the percentage of claims from Canadian suppliers in 2006–2007 was slightly higher than that from U.S. suppliers. Most claims from Canadian suppliers originated in Ontario (Figure 2).
Figure 2: Geographic origin of claims, 2003-2004 to 2006–2007 (average percentages)
Claims Processing
A total of 192 claims for exemptions were processed to completion, which represents a drop of 36% from the number processed in 2005–2006. Much of this decrease from the previous year can be attributed to the unusual complexity of more than half the claims reviewed; they involved 10 ingredients or more and required significantly more time for review. In addition, recently hired staff members were not fully trained, which affected productivity.
In 2006–2007, the Commission updated and enhanced several assessment tools to ensure that MSDS reviews are based on the most advanced scientific expertise. For example, the Commission's prioritization scheme, developed in 2005–2006, was updated to include the most current scientific information on the hazards of workplace chemicals. The Commission developed the prioritization scheme so that claims for products with a high hazard, which are likely to pose a major risk to workers' health, will be identified and reviewed without significant delay. This practice allows the corrected MSDSs of high-hazard products to reach the workplace sooner. Of the 192 decisions issued in 2006–2007, 55% contained ingredients classified as high hazard.
The reference manual used by scientists in reviewing MSDSs was also revised. The Commission's database was updated to include published papers on 528 new ingredients. Eight toxicology profiles were updated as well to include the most recent scientific literature.
The dispute resolution mechanism successfully dealt with 387 issues raised through increased transparency and communication between claimants and the Commission. The majority of the issues resolved dealt with the disclosure on the MSDS of all hazardous ingredients in a product. Another significant type of issue concerned the extent of a product's potential for causing irritation or corrosion to either the skin or the eyes. All of the issues were eventually resolved and no appeals were filed.
MSDS Violations
An MSDS is required to be fully compliant with the Hazardous Products Act and Controlled Products Regulations when a claim is submitted to the Commission. To ensure that this is the case, the Commission reviews the MSDSs for all claims. As in previous years, only about 5% of the MSDSs (10/192) were found to be in compliance, and on average there were 8.3 violation occurrences per claim in 2006–2007 reflecting nearly similar occurrences in the previous two years. Of the great majority of MSDSs that were non-compliant, approximately 59.5% of the violations were related to toxicological properties, hazardous ingredients and first aid measures as indicated by average percentages over the last three years. MSDS non-compliance in these important areas has the potential to negatively impact the health and safety of workers who come in contact with the products involved.
MSDS Violations, 2004–2005 to 2006–2007
| Violation Category | Number of Violations by Year | |||||
|---|---|---|---|---|---|---|
| 2006–2007 | 2005–2006 | 2004–2005 | Total | % | ||
| Toxicological properties | 372 | 850 | 769 | 1991 | 31.5 | |
| Hazardous ingredients | 257 | 333 | 254 | 844 | 13.4 | |
| First aid measures | 249 | 370 | 312 | 931 | 14.7 | |
| Preparation information | 237 | 232 | 147 | 616 | 9.7 | |
| Registry number/date of filing | 59 | 263 | 147 | 469 | 7.4 | |
| Physical data | 92 | 95 | 79 | 266 | 4.2 | |
| Reactivity data | 33 | 117 | 107 | 257 | 4.1 | |
| Hazard classification | 53 | 76 | 80 | 209 | 3.3 | |
| Format/wording | 82 | 57 | 36 | 175 | 2.8 | |
| Fire or explosion hazard | 52 | 58 | 58 | 168 | 2.7 | |
| Headings | 41 | 52 | 70 | 163 | 2.6 | |
| Generic chemical identity | 53 | 43 | 12 | 108 | 1.7 | |
| Product information | 15 | 55 | 28 | 98 | 1.6 | |
| Preventive measures | 6 | 14 | 4 | 24 | 0.4 | |
| Total | 1,601 | 2,615 | 2,103 | 6,319 | 100 | |
| Number of claims/controlled products | 192 | 298 | 245 | 735 | ||
| Average number of violation occurrences per claim | 8.3 | 8.7 | 8.6 | 8.6 | ||
Workload Estimates
Historically, the volume of claims received annually has fluctuated dramatically, making the workload difficult to plan. However, in recent years the number and breakdown of claims received has remained relatively consistent. Consequently, the Commission has established the 2005–2006 and 2006–2007 years as a baseline upon which to forecast the workload for 2007–2008 and subsequent years.
To forecast the number of claims expected to be withdrawn in 2007–2008 and in subsequent years, an average of the last three years (15%) was used as an estimate. The withdrawal of claims can occur for various reasons. For instance, if a company changes hands, the new company must withdraw the claims and refile them; a company may decide to declare the ingredients that it was seeking to protect; or the company may no longer be selling the product. The Commission staff often spend a significant amount of time reviewing these claims before they are withdrawn.
All forecasted numbers are reassessed and adjusted annually if required to ensure that all projections remain meaningful.
Table 2: Claim workload estimates, 2005–2006 to 2008–2009
| Actual number of claims | Estimated number of claims | |||
|---|---|---|---|---|
| 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | |
| Brought forward | 691 | 708 | 813 | 791 |
| PLUS | ||||
| New Claims | 271 | 267 | 280 | 280 |
| Refilings | 117 | 120 | 120 | 120 |
| Subtotal | 388 | 387 | 400 | 400 |
| MINUS | ||||
| Withdrawls | 73 | 90 | 122 | 118 |
| Claims Processed | 298 | 192 | 300 | 300 |
| Subtotal | 371 | 282 | 422 | 418 |
| EQUALS | ||||
| Balance* | 708 | 813 | 791 | 773 |
* Indicates the number of claims remaining to be adjudicated.
Improve Services to Our Clients and Stakeholders
In 1999, following broad consultations with stakeholders and a thorough review of its operations, the Commission embarked on a wide-ranging renewal process to improve the quality and timeliness of its service to clients. Throughout the renewal process the Commission made extensive operational improvements, and with the exception of three matters that required legislative amendment, has successfully implemented all administrative changes that were identified in the extensive consultation and review.
In 2006–2007, the Commission entered the final stage of the renewal process as the three legislative amendments were introduced to Parliament as Bill S-2, An Act to Amend the Hazardous Materials Information Review Act. Bolstered by the unanimous support of all stakeholders and all parties in both the House of Commons and the Senate, Bill S-2 passed without amendment and received Royal Assent on March 29, 2007.
These three amendments, which will be fully implemented in the next fiscal year, will further streamline the Commission's administrative processes for clients. The first amendment relates to the Commission's mandate to protect industry's confidential business information. Currently, claimants seeking to exempt certain information from disclosure are required to submit detailed documentation on how they are protecting the confidentiality of their information and on how they would be harmed by the disclosure of the information. These requirements are an administrative burden on claimants, and also increase the amount of time that the Commission needs to review the claims. The amendment will allow claimants to declare, with minimal supporting documentation, that the information they are seeking to protect from disclosure is confidential business information, and will decrease the Commission's review time. However, the Commission will collect full documentation when an affected party challenges a claim or when a claim is selected for review.
The amendments will also allow claimants to voluntarily correct MSDSs and product labels when the Commission finds that these are non-compliant. The old Act required the Commission to issue formal correction orders against a claimant, even if the claimant was fully prepared to make all necessary corrections voluntarily. Claimants felt that these orders imply a reluctance to fulfill their responsibilities for workplace safety. Additionally, these orders only became binding 75 days after being published in the Canada Gazette. Allowing corrections to be made without issuing orders will dramatically shorten the process and give workers much faster access to accurate safety information.
Finally, the amendments will allow the Commission to provide factual clarification to independent appeal boards, if needed, to facilitate the appeals process. Previously, the Commission was prohibited from providing explanatory information to appeal boards, which often resulted in difficulties for appeal boards when interpreting the record of the screening officer. The amendment to allow factual clarifications will facilitate decision-making by appeal boards and expedite the appeals process.
In summary, the implementation of these amendments will reduce the time required to review claims for exemption from disclosure of confidential information, speed up correction of the information that workers need to handle hazardous materials safely, and expedite the appeals process.
Although the formal renewal process has been successfully concluded, the Commission remains committed to making continuous improvements. In that spirit, both the claims form and the claims process were reviewed, and both will be further enhanced in the next fiscal year. The new electronic claims form will include several interactive elements that will simplify the process for claimants by clarifying what information is needed, organizing claimant input and adjusting to accommodate lengthy input. The new form is also expected to help speed up the processing of claims.
Throughout the year, Commission staff remained committed to excellence in service to prospective claimants seeking information about the claims process and the review of MSDSs. The Commission responded promptly to queries directed to its area of expertise. The Commission was also expedient in responding to queries received from the general public and professionals alike worldwide, involving our partners in occupational health and safety when necessary.
The Commission worked with Health portfolio partners on several executive-level committees. By strengthening ties with the offices of both the Minister and the Deputy Minister of Health, the Commission was able to facilitate the Order-in-Council appointment process for the Council of Governors. Through extensive interaction with the Minister's office, all positions on the Council of Governors that require the Minister of Health's approval have been filled-for the first time in 10 years.
Monitor Implementation of the Globally Harmonized System
The Commission continued to monitor Canada's implementation of the Globally Harmonized System for the Classification and Labelling of Chemicals (GHS), an international initiative issued in 2002 after several years of negotiations, which is scheduled to be implemented worldwide in 2008.
The Commission is involved in several aspects of the GHS as a member of: the tripartite WHMIS Current Issues Committee, which ensures a common understanding of the GHS as it evolves and establishes a Canadian position on these developments across all WHMIS stakeholders; the technical tripartite working group associated with the Current Issues Committee, which is developing consensus approaches for the implementation of the GHS; and the Intergovernmental WHMIS Coordinating Committee, which establishes consensus among the multiple government jurisdictions responsible for WHMIS on the implications for governments arising from the GHS. As progress is made on implementing the GHS in Canada, the Commission can contribute its expertise and experience in MSDS compliance for the benefit of all WHMIS stakeholders.
The Commission has also begun to monitor changes in the trade secret protection mechanisms in other countries and to determine how related GHS provisions are being implemented. By keeping abreast of the evolution of trade secret protection mechanisms globally, the Commission will be able to share best practices from the Canadian experience. It will also be able to ensure that, consistent with the Canadian approach, international efforts to harmonize trade secret protection mechanisms maintain a balance between the protection of worker health and safety and the need for suppliers to protect their trade secrets.
Improve the Focus of Outreach and Stakeholder Liaison
Outreach activities in 2006–2007 focused on the Commission's Web site, the primary outreach and communications tool for both claimants and stakeholders. In 2006–2007, the site recorded 36,180 visitors, an average 34% increase in traffic over the previous year.
Increased interest in the Web site and pending legislative changes prompted a comprehensive Web site review. Commission staff began preparing for three major updates scheduled for the coming year. First, a virtual Web site was developed to reflect the implementation of the new legislative amendments. Second, plans were made to improve how claimants access information on the site. And finally, planning began on how to bring the site into compliance with the Common Look and Feel Standards for the Internet (CLF2). The sites of all federal agencies must meet the new standard by December 31, 2008.
The Commission participated in several industry trade shows in 2006–2007, including two conferences sponsored by the Industrial Accident Prevention Association, the Salon professionnel - Le Grand Rendez-vous, santé et sécurité au travail in Montreal and the Society of Toxicology conference in Charlotte, North Carolina. Such events are key opportunities for the Commission to promote its mandate, role and activities.
In 2006–2007, the Commission reinforced its links with several organizations that have WHMIS-related mandates, including the Canadian Centre for Occupational Health and Safety (CCOHS) and the National Office of WHMIS (NOW) of Health Canada. The Commission, CCOHS and NOW jointly sponsored a national symposium on WHMIS-related issues, where the Commission presented a paper on its mandate and MSDS violations.
Other program:
Financial Resources ($ thousands)
| Planned Spending | Authorities | Actual Spending |
|---|---|---|
| 176 | 180 | 31 |
Dispute Prevention/Appeals
The Dispute Prevention/Appeals process that HMIRC administers had been identified as a second program sub-activity in the 2006–2007 Report on Plans and Priorities. Given the low volume of appeals that have occurred since the inception of the Commission, the internal importance, the size, and the fact that the resources used by this program are not significant, it is not presented as a distinct sub-activity.
For additional information on Dispute Prevention/Appeals, please refer to http://www.hmirc-ccrmd.gc.ca/english/institutional/aboutus.shtml#dispute.
